
Exhibit 99.1


• This document may include statements by PharmaCyte Biotech that constitute “forward - looking statements.” Such statements are often characterized by the terms “may,” “believes,” expects” or “anticipates” and do not reflect facts. • Forward - looking statements involve risks, uncertainties and other factors that may cause actual results, performance or achievements of PharmaCyte and its subsidiaries to be materially different from those expressed or implied by such forward - looking statements. Forward - looking statements speak only as of the date the statement was made. PharmaCyte does not undertake, and specifically declines, any obligation to update any forward - looking statements. • Factors that may affect forward - looking statements and PharmaCyte’s business generally including, but not limited to: (i) the risk factors, cautionary and other statements set forth in PharmaCyte’s periodic filings with the Securities and Exchange Commission available at www.sec.gov, and (ii) other factors that PharmaCyte is currently unable to identify or quantify but may exist in the future. 2 Safe Harbor Statement and Disclaimer

• This presentation shall not constitute an offer to sell or a solicitation of an offer to buy any securities, nor shall there be any sale of such securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction . 3 Safe Harbor Statement and Disclaimer (cont’d)
Encapsulate Genetically Modified Live Cells to Treat Diseases • Cancer : o Encapsulated cells convert a prodrug from its inactive form to its cancer - killing form o Encapsulated cells are implanted near the site of the tumor; low dose chemotherapy prodrug is given intravenously. Encapsulated cells act as an artificial liver to convert the prodrug at the site of the tumor o We believe that this technology results in optimal cytotoxic effect with little to no treatment - related side effects • Diabetes : o Encapsulated cells that produce, store, and release insulin in response to concentrations of glucose in the body are employed o Encapsulated cells are implanted to act as an artificial pancreas for insulin production. 4 Platform Technology for Cancer and Diabetes
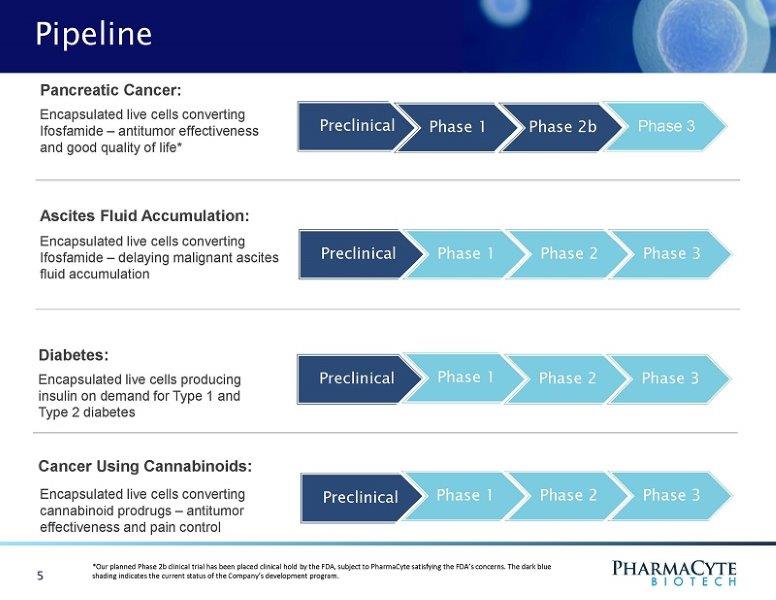
Pipeline Pre cli ni c a l Phase 1 Phase 2b Phase 3 Encapsulated live cells converting Ifosfamide – antitumor effectiveness and good quality of life* Pancreatic Cancer: Preclinical Phase 1 Phase 2 Phase 3 Preclinical Phase 1 Phase 2 Phase 3 Diabetes : Encapsulated live cells producing insulin on demand for Type 1 and Type 2 diabetes Preclinical Phase 1 Phase 2 Phase 3 5 Cancer Using Cannabinoids : Encapsulated live cells converting cannabinoid prodrugs – antitumor effectiveness and pain control Ascites Fluid Accumulation: Encapsulated live cells converting Ifosfamide – delaying malignant ascites fluid accumulation *Our planned Phase 2b clinical trial has been placed clinical hold by the FDA, subject to PharmaCyte satisfying the FDA’s concerns. The dark blue shading indicates the current status of the Company’s development program.
Platform Technology 6 Targeted Chemotherapy for Solid Tumors • Cell - in - a - Box ® encapsulated live cells + cancer prodrug ifosfamide are used • Ifosfamide at its “normal dose” has shown success in treating some cancers, but it cannot be used at the normal dose due to severe toxicity • Cell - in - a - Box ® capsules containing genetically modified live cells that produce an enzyme which converts ifosfamide into its cancer - killing form are implanted in the blood supply near the tumor • Ifosfamide is then administered intravenously at a low dose. Ifosfamide is converted at site of tumor. It is normally converted in the liver • Placement of the Cell - in - a - Box ® capsules near the tumor enables the production of optimal concentrations of the “cancer - killing” form of ifosfamide at the site of the tumor • The cancer - killing metabolite of ifosfamide has a short half - life, which we believe will result in little to no collateral damage to other organs or tissues in the body • We believe this significantly reduces tumor size with little to no treatment - related side effects

Cell - in - a - Box ® Capsules 7 Unique Encapsulation Material • Capsules are made of bio - inert material (cellulose/cotton) • Capsules have pores for nutrient and waste transfer • The pores are too small for immune system cells to enter or encapsulated live cells to leave the capsules • The encapsulated live cells can be stored frozen for long periods (5+ years) and when the cells are thawed, they recover with approximately 85% viability • Manageable logistics and long shelf - life • Other live cell encapsulation technologies use substances such as alginate – derived from seaweed – for the encapsulation material. All are far less robust and stable. None can be frozen to ship the encapsulated live cells
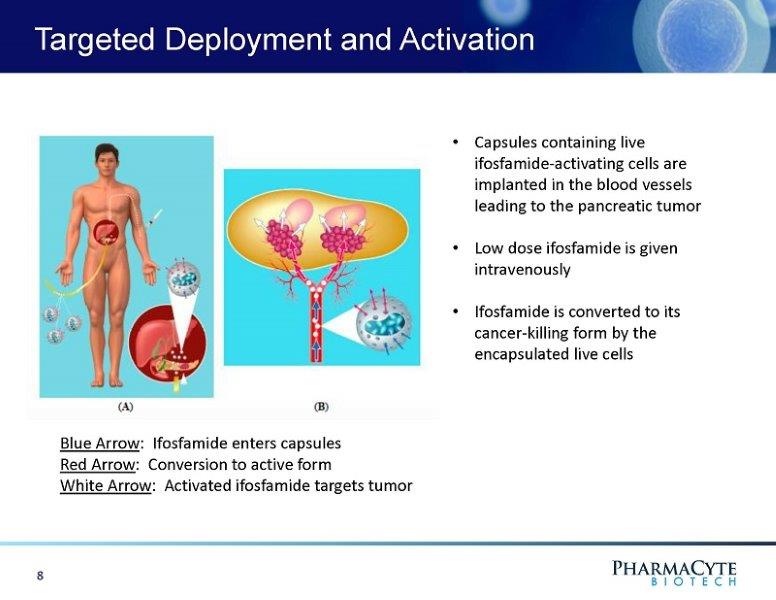
Targeted Deployment and Activation • Capsules containing live ifosfamide - activating cells are implanted in the blood vessels leading to the pancreatic tumor • Low dose ifosfamide is given intravenously • Ifosfamide is converted to its cancer - killing form by the encapsulated live cells 8 Blue Arrow : Ifosfamide enters capsules Red Arrow : Conversion to active form White Arrow : Activated ifosfamide targets tumor

Mechanism of Action Immune system cells too large to enter capsule Single capsule containing ifosfamide - activating live cells Inactive ifosphamide “prodrug” “Activated” ifosfamide molecules cancer killing in - situ P anc r e as tumor 9

Placement of Encapsulated Cells A. Angiography of blood vessels to the pancreas B. Insertion of catheter into the pancreas blood vessel (arrow) C. Injection of microcapsules D. Angiography shortly after capsule implantation (arrow) Blood supply to the pancreas is not impeded by the capsules 10
Pancreatic Cancer Aggressive Cancer with Poor Prognosis • Increasing in most industrialized countries • Third leading cause of cancer - related deaths in the western world • Expected pancreatic cancer patients in 2021: U.S. >60,430; 48,480 deaths • Approximately 80% die within the first year of diagnosis • The five - year survival rate for Stage 4 pancreatic cancer is approximately 3% • The survival rate is approximately 2% after 10 years from diagnosis • Without treatment, pancreatic cancer patients have 3 - 6 months to live • Usually not diagnosed until cancer is advanced and inoperable • No cure unless surgically removed in earliest stages; only 15% are operable • Since the first drug (gemcitabine) was approved for pancreatic cancer in 1996, approximately 40 pivotal Phase 3 clinical trials have been conducted • Little improvement in median survival time and percentage of 1 - year survivors • Most success has been achieved with gemcitabine + another chemotherapy drug 11

Pancreatic Cancer (cont’d) 12 Current First Line Therapy Abraxane ® + Gemcitabine • Combination approved by FDA in September 2013 • Increased median survival by 1.8 months as compared to gemcitabine alone • Increased the percentage of one - year survivors from 22% with gemcitabine to 38% with Abraxane ® + gemcitabine • Severe side effects from Abraxane ® + gemcitabine therapy FOLFIRINOX • A combination of 4 drugs – folinic acid, 5 - fluorouracil, irinotecan and oxaliplatin • Phase 3 clinical trial done in France. Never received marketing approval • Should only be used in otherwise healthy patients • Severe side effects from FOLFIRINOX therapy

Trials in Pancreatic Cancer Using Our Product 13 Candidate Phase 1/2 Clinical Trial with Two Courses of Low Dose Ifosfamide (1998 - 1999) • Fourteen patients were treated with only two courses of ifosfamide at 1/3 (1 g/m) of the dose normally used to treat other forms of cancer • Median survival : gemcitabine = 5.7 months vs. Cell - in - a - Box ® + ifosfamide = 10 months • Percentage of 1 - year survivors : gemcitabine = 18% vs. Cell - in - a - Box ® + ifosfamide =36% • Treatment - related side effects : gemcitabine = significant vs. Cell - in - a - Box ® + ifosfamide = none Phase 2 Clinical Trial with Two Courses of Twice the Amount of Ifosfamide (1999 - 2000) • Thirteen patients with advanced, inoperable pancreas cancer were treated in a single - arm, multi - site (3 in Germany [Rostock, Berlin, Munich], 1 in Berne, Switzerland) study. The only difference from the Phase 1/2 trial was that the dose of ifosfamide was doubled to 2 g/m 2 in an attempt to get better antitumor effects • Doubling the dose of ifosfamide did not result in greater antitumor effectiveness, but resulted in treatment - related side effects Overall Comparison of Results of the Two Clinical Trials • When used in combination with Cell - in - a - Box ® capsules, ifosfamide should be given at a low doses to maximize anti - tumor effect and eliminate side effects
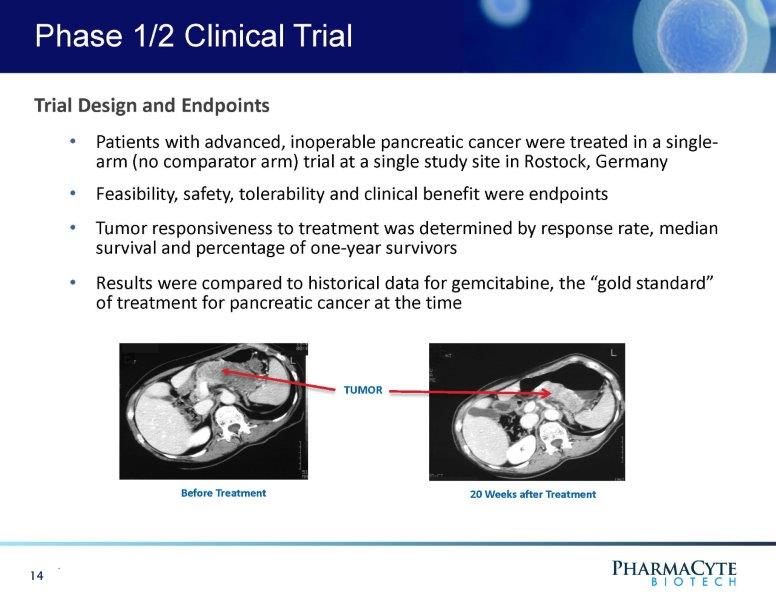
Phase 1/2 Clinical Trial . 14 Trial Design and Endpoints • Patients with advanced, inoperable pancreatic cancer were treated in a single - arm (no comparator arm) trial at a single study site in Rostock, Germany • Feasibility, safety, tolerability and clinical benefit were endpoints • Tumor responsiveness to treatment was determined by response rate, median survival and percentage of one - year survivors • Results were compared to historical data for gemcitabine, the “gold standard” of treatment for pancreatic cancer at the time Before Treatment 20 Weeks after Treatment TU M OR

Planned Trial in Pancreatic Cancer 15 Addressing Critical Unmet Medical Need • A critical unmet medical need exists for patients with pancreatic cancer whose tumors are locally advanced, non - metastatic and inoperable but no longer respond to Abraxane ® + gemcitabine or FOLFIRINOX • These patients have no effective treatment alternative once their tumors no longer respond to these combination therapies • The two most commonly used treatments for these patients are 5 - fluorouracil (5 - FU) or capecitabine (a prodrug of 5 - FU) chemotherapy + radiation or radiation alone • Both treatments are marginally effective in treating the tumor and result in serious side effects • The goal of our planned Phase 2b clinical trial is to show that PharmaCyte’s product candiate for pancreatic cancer can serve as a “consolidation therapy” with Abraxane ® + gemcitabine or FOLFIRINOX and address the unmet medical need for these pancreatic cancer patients
Trial Design 16 Elements of Trial Design • Design: Trial will be two - armed • Location: Trial will be conducted in the U.S. • Objective: Trial is designed to show Cell - in - a - Box ® + low - dose ifosfamide can serve as an effective and safe consolidation chemotherapy for patients whose tumors no longer respond to the therapy of Abraxane ® + gemcitabine or FOLFIRINOX after 4 - 6 months of treatment • CRO Administration: Trial will be conducted in the U.S. by Medpace • CRO Responsibilities: Clinical development plans, program analysis, medical writing, clinical management and database development • Radiologist Responsibilities: Coordinate implanting the Cell - in - a - Box ® capsules and all measurements of antitumor effectiveness of the therapy as measured by CT scans • Start Date: Trial is expected to start in Q3 of 2022, subject to the FDA lifting the clinical hold • Eligibility: Only patients whose tumors are locally advanced, inoperable and non - metastatic will be eligible to be enrolled
Trial Design (cont’d) 17 • Eligibility: Patients must have been treated with Abraxane ® + gemcitabine or FOLFORINOX for 4 - 6 months until their tumors no longer respond to this therapy or the therapy is refused • Randomization: 100 patients will be randomized into two groups. 50 patients will receive PharmaCyte’s pancreatic cancer candidate. The other 50 patients will receive either capecitabine + external beam radiation therapy (EBRT) or stereotactic body radiation therapy (SBRT) alone • PharmaCyte Treatment Group: Each patient treated with PharmaCyte’s therapy will receive a single implantation of 300 Cell - in - a - Box ® capsules + multiple courses of low - dose ifosfamide until the patient becomes refractory or there is an unacceptable toxicity level from the treatment • Primary Endpoints: Progression - free survival measured from randomization to disease progression or death
Secondary Endpoints: • Overall - survival is evaluated as the time from randomization to death by any cause • Objective response rate is evaluated as the combined incidence of complete response (CR) and partial response (PR) • Conversion from inoperable at randomization to operable post - treatment is evaluated by the proportion of patients who convert to resectable (RO/R1) disease • Change in CA19 - 9 biomarker level from screening/baseline • Time to onset of pain and pain management • Assessment of patients’ overall quality - of - life while undergoing PharmaCyte’s product candidate • Safety will be assessed for the overall frequency of adverse events by measuring vital signs, 12 lead EKG, serum antibody response in patients receiving PharmaCyte’s therapy, physical examinations and clinical laboratory test results 18 Trial Design (cont’d)

Trial Design Oncologists Leading Experts in Development of Therapies to Treat Pancreatic Cancer Dr. Daniel D. Von Hoff 19 Dr. Manuel Hidalgo Dr. Matthias Löhr

Trial Design Oncologists (cont’d) 20 Dr. Daniel Von Hoff • A world’s leading oncologist in the development of drugs to treat pancreas cancer • Involved in clinical trials of more than 200 anticancer and biologic drugs • Conducted early clinical trials for most of the cancer agents approved in the U.S. in the last 20 years • Intimately involved in the clinical development of gemcitabine and Abraxane ® for pancreas cancer • Editor of numerous oncologic scientific journals; recipient of numerous awards for cancer - related activities • Professor of Medicine at Mayo Clinic Scottsdale and University of Arizona College of Medicine, Chief Scientific Officer of Scottsdale Healthcare and U.S. Oncology, Physician - in - Chief and Distinguished Professor of the Translational Genomics Research Institute (TGen) and Chief Development Officer of Translational Drug Development (TD2) Dr. Manuel Hidalgo • Internationally - renowned oncologist and clinical investigator in pancreas and other cancers • Co - founder and Chairman of the International Pancreatic Cancer Research Team • Assisted in the development of more than 30 novel oncology drugs; several for pancreas cancer • Former head of Clinical Development at the Spanish National Cancer Research Center in Madrid and Co - Director of Drug Development and Gastrointestinal Oncology at Johns Hopkins University • Former Clinical Director of the Rosenberg Clinical Cancer Center and Chief of the Division of Hematology - Oncology at Beth Israel Deaconess Medical Center in Boston • Chief of the Division of Hematology and Medical Oncology/Weill Cornell Medicine and NewYork Presbyterian/Weill Cornell Medical Center Dr. Matthias Löhr • One of Europe’s leading authority in diseases of the pancreas (pancreas cancer and diabetes) • Has published many important articles/commentaries on pancreas cancer and use of the Cell - in - a - Box ® technology • Principal Investigator for previous clinical trials of PharmaCyte’s pancreas cancer therapy • Chairman of PharmaCyte’s Medical and Scientific Advisory Board • Currently Professor of Gastroenterology and Hepatology at Sweden’s famed Karolinska Institute

Trial Preparations 21 Manufacturing Capability • GMP facility successfully audited and deemed ready for production of GMP clinical trial product, known as “CypCaps Ρ ” • Two staggered and back - to - back manufacturing runs of clinical trial successfully completed • FDA required “release testing” completed and passed tests on CypCaps Ρ • Successful Stability Study for 3, 6, 9, and 12 - month timepoints • CypCaps Ρ expected to be released into the U.S. when needed Trial Preparations • Pre - IND meeting held with FDA (January 2017) • FDA identified numerous tests to be conducted and data to be developed to support a successful IND submission • Met with US/EU investigators at ASCO annual meetings to refine trial design

• Dr. Manuel Hidalgo selected as Principal Investigator • Protocol and Investigator Brochure finalized, subject to input from the FDA • Practical Clinical retained to function as the Director of Clinical Operations • Medpace selected as CRO to conduct the trial. Medpace is among the top 10 CROs in the world and a full - service CRO with expertise in numerous therapeutic areas focused on supporting the biotech sector, particularly pancreatic cancer • Prepared Angiography Guidelines • Prepared Pharmacy Manual • Selection process for vendor drug supply chain and storage completed 22 Trial Preparations (cont’d)

IND Submission to FDA • IND filed with FDA on September 1, 2020, to commence Phase 2b clinical trial in locally advanced, inoperable pancreatic cancer (LAPC) • On October 1, 2020, received notice that the FDA had placed the IND on clinical hold • On October 30, 2020, FDA sent a letter to PharmaCyte setting forth the reasons for the clinical hold and providing specific guidance on what we must do to have the clinical hold lifted The Clinical Hold • The list of items PharmaCyte must complete to have the clinical hold lifted is set forth in Appendix A • Assembled a scientific and regulatory team of experts to address the FDA concerns • Team is working to complete the list of items the FDA required of us. In varying stages of addressing the studies and acquiring the information requested by the FDA. A summary of our work is set forth in Appendix A 23 Investigational New Drug Application

FDA Granted Orphan Drug Designation for Pancreas Cancer Therapy • Provides 7 years of market exclusivity in the U.S. upon FDA approval EMA Granted Orphan Drug Designation for Pancreas Cancer Therapy • Provides 10 years of market exclusivity in the E.U. upon EMA approval Eligible for Biologics Price Competition and Innovation Act • Provides 12 years of market exclusivity in the U.S upon FDA approval. Similar laws in the E.U. provide market exclusivity IP Portfolio and IP Protection Strategy • Patent applications filed in the U.S., Australia and Canada to protect the genetically engineered cells that convert cancer prodrug for patient population • Exclusive license world - wide for cancer therapy and diabetes therapy using encapsulated genetically modified human cells • Patents protect the Melligen cells • Follow - on patents expected to be filed for each product candidate • Encapsulation process uses unique patent - protected cellulose sulphate • Trade secrets and know - how 24 Orphan Drug Status and IP Portfolio

Malignant Ascites Fluid Accumulation 25 Targeted Chemotherapy to Treat Malignant Ascites • Malignant ascites fluid is secreted by abdominal tumors into the abdomen • Contains cancer cells that can seed and form new tumors throughout the abdomen • Accumulates in the abdominal cavity causing swelling of the abdomen, severe breathing difficulties and extreme pain • Must be removed on a periodic basis - this is painful and costly • No available therapy prevents or delays the production and accumulation of malignant ascites fluid • Translational Drug Development (TD2) has conducted 8 preclinical studies to determine if PharmaCyte’s cancer therapy can delay the production and accumulation of malignant ascites fluid from abdominal cancers o Data indicated the treatment might play such a role, but the conclusions were difficult to interpret with certainty • PharmaCyte plans to conduct another preclinical study in Germany to determine if the TD2 conclusions are valid • If successful, plan to seek FDA approval to conduct a Phase 1 study in U.S.

Bio - Artificial Pancreas for Diabetes • PharmaCyte’s Diabetes Program consists of encapsulating genetically modified cells (Melligen cells and stem cells) that produce insulin in proportion to a patient’s blood glucose level and then implanting the capsules in the body to treat Type 1 diabetes and insulin - dependent Type 2 diabetes • The Cell - in - a - Box® capsules protect the cells from immune system attack in the body and thus they function as a “bio - artificial pancreas” for the purpose of insulin production • PharmaCyte has the exclusive world - wide license to use Melligen insulin - producing cells to treat diabetes o Melligen cells are human liver cells that have been genetically modified to produce, store and release insulin in response to concentrations of glucose in the body o Melligen cells have demonstrated the ability to reverse the diabetic condition in immunosuppressed diabetic mice 26 Diabetes Program

o In the past, PharmaCyte’s International Diabetes Consortium encountered difficulties related to the stability of the Melligen cells o PharmaCyte then spent two years recreating a stable version of the Melligen cells • UTS entered into an agreement with PharmaCyte to create a new, stable and advanced version of the Melligen cells • Improvements will also be made to increase the insulin production of the Melligen cells and the bioactivity of the produced insulin • \ Diabetes Program (cont’d) 27
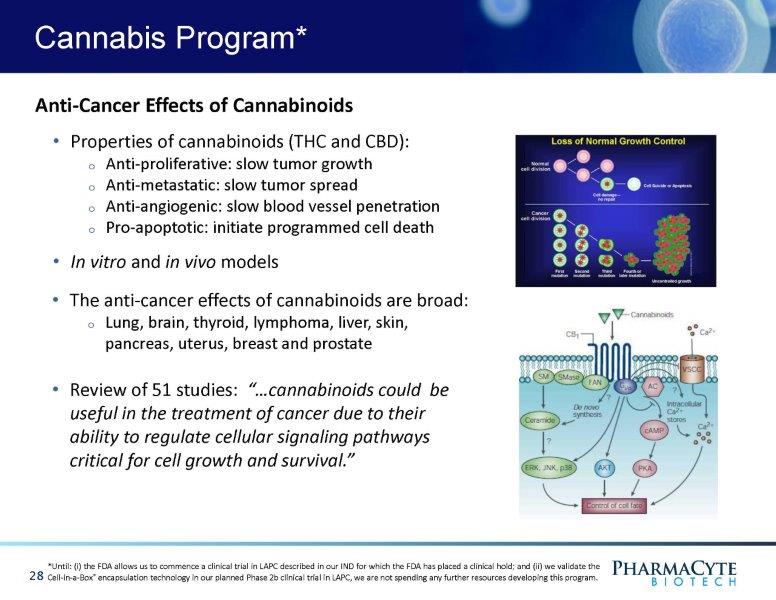
Cannabis Program* Anti - Cancer Effects of Cannabinoids • Properties of cannabinoids (THC and CBD): o Anti - proliferative: slow tumor growth o Anti - metastatic: slow tumor spread o Anti - angiogenic: slow blood vessel penetration o Pro - apoptotic: initiate programmed cell death • In vitro and in vivo models • The anti - cancer effects of cannabinoids are broad: o Lung, brain, thyroid, lymphoma, liver, skin, pancreas, uterus, breast and prostate • Review of 51 studies: “…cannabinoids could be useful in the treatment of cancer due to their ability to regulate cellular signaling pathways critical for cell growth and survival.” *Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; and (ii) we validate the Cell - in - a - Box ® encapsulation technology in our planned Phase 2b clinical trial in LAPC, we are not spending any further resources developing this program. 28
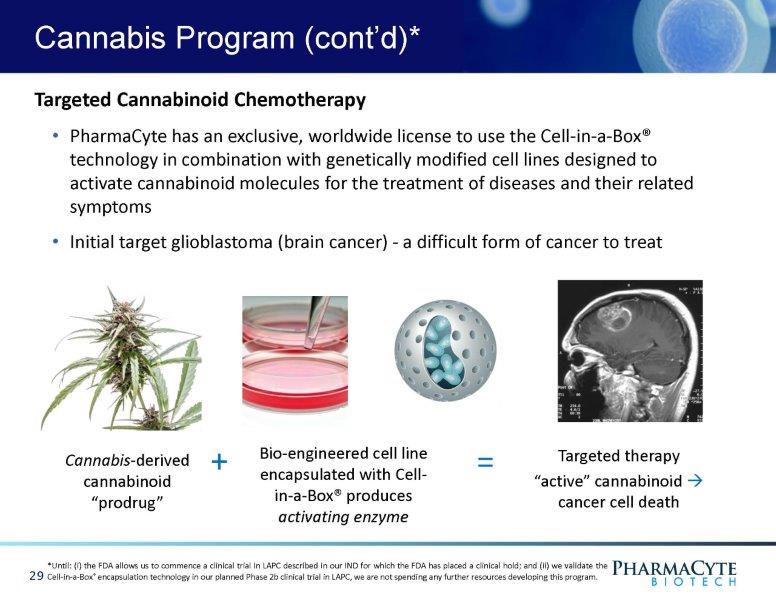
Cannabis Program (cont’d)* Cannabi s - d er i v ed cannabinoid “prodrug” Bio - engineered cell line encapsulated with Cell - in - a - Box® produces activating enzyme Targeted therapy “active” cannabinoid • cancer cell death Targeted Cannabinoid Chemotherapy • PharmaCyte has an exclusive, worldwide license to use the Cell - in - a - Box® technology in combination with genetically modified cell lines designed to activate cannabinoid molecules for the treatment of diseases and their related symptoms • Initial target glioblastoma (brain cancer) - a difficult form of cancer to treat + *Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; and (ii) we validate the Cell - in - a - Box ® encapsulation technology in our planned Phase 2b clinical trial in LAPC, we are not spending any further resources developing this program. 29 =

Research Program with University of Northern Colorado • Initial goal was to develop methods for identification, separation and quantification of constituents of Cannabis which are prodrugs requiring activation. Methods have now been developed • The focus of this aspect of the Cannabis Program is: o Confirming the anti - cancer activity of cannabinoids o Developing a cell capable of converting an inactive cannabinoid prodrug into its cancer - killing form. UTS has now genetically engineered such a cell • Testing the efficiency of the transfected cells in converting cannabinoid prodrugs into their active cancer - killing forms • Targeted cannabinoid - based chemotherapy would be accomplished by implanting the bio - engineered cells near the site of the tumor along with administration of a cannabinoid prodrug *Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; and (ii) we validate the Cell - in - a - Box ® encapsulation technology in our planned Phase 2b clinical trial in LAPC, we are not spending any further resources developing this program. 30 Cannabis Program (cont’d)

Development of Pain Therapy • Chronic pain linked to restricted mobility, opioid dependency, anxiety, depression and reduced quality of life • Estimated $560 billion annually in direct costs, lost productivity and disability programs in U.S. • Additional goal of Cannabis Program is to utilize Cell - in - a - Box ® platform to deliver cannabinoid end - products to provide chronic pain relief program. Cannabis Program (cont’d)* 31 *Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; and (ii) we validate the Cell - in - a - Box ® encapsulation technology in our planned Phase 2b clinical trial in LAPC, we are not spending any further resources developing this

Kenneth L. Waggoner, J.D. – Chief Executive Officer, President and General Counsel • Over four decades of experience in law, management, operations and business • Senior partner with Brobeck, Phleger and Harrison, one of the top law firms worldwide providing services to biotechnology clients such as Chiron, Amgen, Biogen and Idec • Represented Fortune 100 companies most of his professional career Dr. Gerald W. Crabtree – Chief Scientific Officer • Over 50 years experience in cancer research and all phases of cancer drug development • Particularly experienced in preclinical studies and clinical trials of cancer drugs • Led preclinical development of Taxol - a multibillion dollar drug for Bristol - Myers Squibb Dr. José L. Iglesias – Chief Medical Officer for Clinical Trial in Pancreatic Cancer • Key positions with Eli Lilly, Amgen, Abraxis, and Celgene • Served as global Vice - President of Clinical Development at Celgene • Led the team that obtained FDA approval for Abraxane ® Carlos A. Trujillo, CPA – Chief Financial Officer • Has 36 years of experience in finance, accounting and management • For the last thirteen years, he has been the Chief Financial Officer for privately held, publicly traded and multinational companies 32 Leadership Team

Medical and Scientific Advisory Board 33 Dr. Matthias Löhr (Chairman ) • A world - renowned European oncologist/gastroenterologist at the Karolinska Institute in Stockholm, Sweden. Expert in the treatment of pancreas cancer and diabetes • Served as Principal Investigator for the Phase 1/2 and Phase 2 clinical trials of PharmaCyte’s pancreas cancer therapy Dr. Manuel Hidalgo • Internationally renowned oncologist and clinical investigator in pancreatic and other cancers • Co - founder and Chairman of the international Pancreatic Cancer Research Team • Assisted in the development of more than 30 novel oncology drugs • Chief of the Division of Hematology and Medical Oncology at Weill Cornell Medicine and NewYork - Presbyterian/Weill Cornell Medical Center Dr. Brian Salmons • Co - inventor of the Cell - in - a - Box ® live cell encapsulation technology • President and CEO of Austrianova • Accomplished scientist with over 120 publications in scientific journals

Dr. Mark L. Rabe • Leader in use of cannabis to treat diseases and their symptoms • Served as Chief Medical Officer of California’s largest network of physician - owned cannabis evaluation centers David A. Judd • Cellular biologist with the Grand Island Biological Company for over 30 years with particular expertise working with the cell line used in our pancreatic cancer therapy • Has decades of experience culturing difficult to grow cells and troubleshooting cell growth in cell culture media 34 Medical and Scientific Advisory Board (cont’d
FDA Guidance to Have Clinical Hold Lifted • Provide additional sequencing data and genetic stability studies • Conduct stability study on CypCaps Ρ and cells from MCB • Evaluate the compatibility of the delivery devices (prefilled syringes and microcatheter used to implant CypCaps Ρ ) with our drug candidate • Provide additional detailed description of the manufacturing process • Provide additional CypCaps Ρ release specifications • Demonstrate comparability between 1 st generation (CapCells®) and 2 nd generation (CypCaps Ρ ) of drug candidates and ensure adequate and consistent product performance and safety between the two generations of drug candidates • Conduct biocompatibility assessment using the final finished capsules but without cells • Address insufficiencies in Chemistry, Manufacturing and Controls information in the cross - referenced Drug Master File • Conduct an additional nonclinical study to assess the safety, activity and distribution of CypCaps Ρ 35 Appendix A

• Revise Investigators Brochure to include the preclinical studies conducted in response to the clinical hold and remove any statements not supported by the data • Provide data from new pig toxicology study The FDA requested we address the following issues as an amendment to the IND • Provide a Certificate of Analysis for pc3/2B1 plasmid that includes tests for assessing purity, safety and potency • Perform qualification studies for CypCaps filling process to ensure that the drug candidate remains sterile and stable during the process • Submit an updated batch analysis for the drug candidate for the specific lot that will be used for manufacturing of all future drug candidates • Provide additional details for the methodology for Resorufin (CYP2B1) potency and PrestoBlue metabolic assays • Provide a few examples of common microcatheters that fit the specifications in our Angiography Procedure Manual • Clarify the language in the Pharmacy Manual regarding proper use of the syringe fill with drug product • Provide discussion with data for trial of the potential for cellular and humoral immune reactivity against the CYP 2 B 1 protein in an animal study and potential for induction of autoimmune mediated toxicities in our trial population 36 Appendix A (cont’d)
Scientific and Regulatory Team of Experts Assembled a scientific and regulatory team of experts to address FDA’s requests. Working to complete the items requested by FDA. At varying stages of addressing the studies and information requested by the FDA • Successfully completed a 3, 6, 9 and 12 - month stability study on CypCaps Ρ . Next timepoint is 18 months • Designed and commenced studies required by FDA including (i) a stability study on cells from our Master Cell Bank (MCB) used to manufacture CypCaps Ρ ; (ii) sequence analysis of DNA encoding of the CYP2B1 gene in CypCaps Ρ ; and (iii) reproducibility and quality of the filling of the MCB cells into vials ready for manufacture of CypCaps Ρ • Designed and, in some cases, commenced biocompatibility studies: (i) Acute Systematic Toxicity study; (ii) Ames test [Genotoxicity Bacteria and Reverse Mutation tests; (iii) Subchronic and Chronic study; (iv) Skin Sensitization study; (v) Complement Activation test; (vi) Intracutaneous test; (vii) Hemolysis test; (viii) In Vitro Cytotoxicity test; and (ix) In Vivo Micronucleus assay 37 Appendix A (cont’d)
• Austrianova manufactured and delivered 400 syringes of empty capsules to laboratory conducting biocompatibility studies • Designed and commenced studies designed to show CypCaps Ρ were not adversely affected by catheters used by interventional radiologists to deliver CypCaps Ρ nor by contrast medium used to visualize blood vessels during implantation of CypCaps Ρ • Designed and commenced studies to demonstrate how robust the clinical trial product is during delivery and use as well as to document that the syringes used to deliver CypCaps Ρ allow delivery consistently, smoothly and safely • With our support, Austrianova is providing additional confidential information to the FDA on the manufacturing process of CypCaps Ρ , including information on improvements made to the live cell encapsulated product since the last clinical trials in Europe with respect to reproducibility and safety of CypCaps Ρ 38 Appendix A (cont’d)
• In the process of updating our IND submission documents to include (i) more pre - clinical data; (ii) additional parameters for release of CypCaps Ρ ; (iii) recommendation of the catheters and contrast medium to be used to deliver CypCaps Ρ ; and discussion of the potential for cellular and humoral immune reactivity against the CYP2B1 protein and potential for induction of autoimmune mediated toxicities in our clinical trial population • Designed an abbreviated study in pigs to address biocompatibility and the effects of long - term implantation of the capsules • Developed arguments to support our position that we should not be required to conduct a carcinogenicity study required by FDA 39 Appendix A (cont’d)

Contact Investor Relations for Further Information ( InvestorRelations@PharmaCyte.com ) Thank you Investor Relations 40