
Filed Pursuant to Rule 424(b)(5)
Registration No. 333-255044
The information in this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities and we are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED AUGUST 2, 2021
PRELIMINARY PROSPECTUS SUPPLEMENT
(To Prospectus dated April 14, 2021)

Shares of Common Stock
Pre-funded Warrants to Purchase up to Shares of Common Stock
Common Warrants to Purchase up to Shares of Common Stock
Underwriter Warrants to Purchase Shares of Common Stock
We are offering shares of our common stock, par value $0.0001 per share, and common warrants to purchase up to shares of our common stock (the “Common Warrants”) pursuant to this prospectus supplement and the accompanying prospectus. The public offering price for each share of common stock and accompanying Common Warrant to purchase one share of common stock is $ .. The Common Warrants have an exercise price of $ per share, are exercisable immediately and will expire years from the date of issuance. We are also offering the shares of our common stock that are issuable from time to time upon exercise of the Common Warrants.
We are also offering pre-funded warrants (the “Pre-funded Warrants”) to purchase up to an aggregate of shares of common stock (and the shares of common stock issuable from time to time upon exercise of the Pre-funded Warrants), in lieu of shares of common stock, to those purchasers whose purchase of shares of common stock in this offering would result in the purchaser, together with its affiliates and certain related parties, beneficially owning more than 4.99% (or, at the election of the holder, 9.99%) of our outstanding shares of common stock following the consummation of this offering. A holder of Pre-funded Warrants will not have the right to exercise any portion of its Pre-funded Warrants if the holder, together with its affiliates and certain related parties, would beneficially own in excess of 4.99% (or, at the election of the holder, 9.99%) of the number of shares of common stock outstanding immediately after giving effect to such exercise. Each Pre-funded Warrant will be exercisable for one share of common stock at an exercise price of $0.001 per share of common stock. The public offering price is $ per Pre-funded Warrant and accompanying Common Warrant, which is equal to the public offering price per share of common stock and accompanying Common Warrant less $0.001. Each Pre-funded Warrant will be exercisable upon issuance and will expire when exercised in full. The shares of common stock or Pre-funded Warrants, as applicable, and the accompanying Common Warrants, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance. There is no established public trading market for the Pre-funded Warrants or the Common Warrants, and we do not expect a market to develop. We do not intend to apply for listing of the Pre-funded Warrants or the Common Warrants on any securities exchange or nationally recognized trading system. Without an active trading market, the liquidity of the Pre-funded Warrants and the Common Warrants will be limited.
The Company’s OTCQB Market Symbol is PMCB, although as a result of the recent reverse split of the Company’s common stock, until August 6, 2021, the Company’s OTCQB Market Symbol has temporarily been PMCBD. On July 30, 2021, the last reported sale price of our common stock was $12.01 per share.
Our common stock has been approved for listing on the Nasdaq Capital Market (“Nasdaq”) under the symbol “PMCB.”
Except as otherwise indicated, all share and per share information in this prospectus supplement gives effect to the reverse stock split of the Company’s outstanding common stock, which was effected at a ratio of 1-for-1,500 shares as of 12:01am Eastern Time on Monday, July 12, 2021. However, share and per share amounts in the accompanying prospectus and certain of the documents incorporated by reference herein have not been adjusted to give effect to the reverse stock split.
The public offering price per share of common stock, the public offering price per Pre-Funded Warrant and the public offering price per Common Warrant will be determined between us, the underwriter, and investors based on market conditions at the time of pricing, and may be at a discount to the current market price of our shares of common stock. Prior to this offering, there has been a limited public market for our common stock on the OTCQB.
Investing in our securities involves significant risks. Please read the information contained in or incorporated by reference under the heading “Risk Factors” beginning on page S-15 of this prospectus supplement and under similar headings in other documents filed after the date hereof and incorporated by reference into this prospectus supplement and the accompanying prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
|
Per Share and Accompanying Warrant |
Per Pre-funded Warrant and Common |
Total | |||||
| Public offering price(1) | $ | $ | |||||
| Underwriting discounts and commissions(2) | $ | $ | |||||
| Proceeds, before expenses, to us | $ | $ | |||||
| (1) | Includes $ per warrant for the accompanying Common Warrants. |
| (2) | In addition, we have agreed to pay the underwriter a management fee equal to 1.0% of the gross proceeds of this offering, to issue to the underwriter or its designees warrants to purchase shares of common stock equal to 7.5% of the shares issued in this offering (including the shares of common stock issuable upon the exercise of the Pre-funded Warrants) and to reimburse certain expenses of the underwriter in connection with this offering. This prospectus supplement and the accompanying prospectus also covers the underwriter warrants and the shares of our common stock issuable upon exercise of the underwriter warrants. See “Underwriting” for additional disclosure regarding underwriting compensation. See “Underwriting” beginning on page S-40 for additional disclosure regarding the compensation payable to the underwriter. |
We have granted the underwriter an option for a period of up to 30 days from the date of this prospectus supplement to purchase up to additional shares of our common stock at the public offering price of $ , and/or Common Warrants to purchase up to shares of our common stock at the public offering price of $ , less underwriting discounts and commissions. If the underwriter exercises the option in full, the total underwriting discounts and commissions payable by us will be $ and the total proceeds to us, before expenses, will be $ , excluding potential proceeds from the exercise of the Common Warrants included in such option.
Delivery of the shares of common stock, Pre-Funded Warrants and Common Warrants being offered pursuant to this prospectus supplement and the accompanying prospectus is expected to be made on or about , 2021, subject to the satisfaction of certain closing conditions.
Sole Book-Running Manager
H.C. WAINWRIGHT & CO.
The date of this prospectus supplement is August__, 2021.
TABLE OF CONTENTS
Prospectus Supplement
Prospectus
| S-i |
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus are part of a shelf registration statement on Form S-3 (File No. 333-255044) that we filed with the Securities and Exchange Commission, or SEC, on April 5, 2021 and that was declared effective by the SEC on April 14, 2021, pursuant to which we may from time to time offer various securities in one or more offerings.
This document is in two parts. The first part is this prospectus supplement, which describes the terms of this offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference herein or therein. The second part, the accompanying prospectus, including the documents incorporated by reference into the accompanying prospectus, provides more general information. When we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement and the information contained in the accompanying prospectus or any document incorporated by reference herein or therein filed prior to the date of this prospectus supplement, you should rely on the information in this prospectus supplement; provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date - for example, a document incorporated by reference in the accompanying prospectus - the statement in the document having the later date modifies or supersedes the earlier statement.
We have not and the underwriter has not authorized anyone to provide information different from that contained in this prospectus supplement and the accompanying prospectus that we have authorized for use in this offering. If anyone provides you with different or inconsistent information, you should not rely on it. We do not and the underwriter does not take any responsibility for, and neither we nor the underwriter can provide assurance as to the reliability of, any other information that others may give you. Neither the delivery of this prospectus supplement and the accompanying prospectus, nor the sale of our common stock, Common Warrants and Pre-funded Warrants means that information contained in this prospectus supplement and the accompanying prospectus, is correct after their respective dates. It is important for you to read and consider all information contained in this prospectus supplement and the accompanying prospectus, including the information incorporated by reference into this prospectus supplement and the accompanying prospectus in making your investment decision.
This prospectus supplement does not contain all of the information that is important to you. You should also read and consider the information in the documents to which we have referred you in the sections entitled “Where You Can Find More Information” and “Incorporation of Certain Information by Reference” in this prospectus supplement. You should rely only on the information contained or incorporated by reference in this document. You should assume that the information in this prospectus supplement and the accompanying prospectus, as well as the information we have filed with the SEC and incorporated by reference in this document, is accurate only as of its date or the date which is specified in those documents.
We are offering to sell, and seeking offers to buy, and the underwriter is soliciting offers to buy, these securities only in jurisdictions where offers and sales are permitted. The distribution of this prospectus supplement and the accompanying prospectus and the offering of the securities in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement and the accompanying prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities and the distribution of this prospectus supplement and the accompanying prospectus outside the United States. This prospectus supplement and the accompanying prospectus do not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement and the accompanying prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
This prospectus supplement and the information incorporated herein by reference include trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference herein are the property of their respective owners.
Unless the context otherwise requires, in this prospectus supplement the “Company,” “we,” “us,” “our” and similar names refer to PharmaCyte Biotech, Inc. and its subsidiaries.
| S-ii |
This summary highlights certain information about us, this offering and selected information contained elsewhere in or incorporated by reference into this prospectus supplement and the accompanying prospectus. This summary is not complete and does not contain all of the information that you should consider before deciding whether to invest in our common stock, Common Warrants and Pre-funded Warrants. For a more complete understanding of the Company and this offering, we encourage you to read and consider carefully the more detailed information in this prospectus supplement and the accompanying prospectus, including the information incorporated by reference in this prospectus supplement and the accompanying prospectus, including the information referred to under the heading “Risk Factors” in this prospectus supplement beginning on page S-15.
Overview
We are a biotechnology company focused on developing cellular therapies for cancer and diabetes based upon a proprietary cellulose-based live cell encapsulation technology known as “Cell-in-a-Box®..” The Cell-in-a-Box® technology is intended to be used as a platform upon which therapies for several types of cancer, including locally advanced, inoperable, non-metastatic pancreatic cancer (“LAPC”) will be developed. The current generation of our product candidate is referred to as “CypCaps™.” On September 1, 2020, we submitted an Investigational New Drug Application (“IND”) to the U.S. Food and Drug Administration (“FDA”) for a planned Phase 2b clinical trial in LAPC. On October 1, 2020, the Company received notice from the FDA that it had placed the IND on clinical hold. On October 30, 2020, the FDA sent a letter to us setting forth the reasons for the clinical hold and specific guidance on what we must do to have the clinical hold lifted. To lift the clinical hold, the FDA has informed us that we need to conduct several additional preclinical studies and assays. The FDA also requested additional information regarding several topics, including DNA sequencing data, manufacturing information and product release specifications. We are in the process of conducting these studies and assays and gathering additional information to submit to the FDA. See “Our Investigational New Drug Application and the Clinical Hold” below.
The Cell-in-a-Box® encapsulation technology potentially enables genetically engineered live human cells to be used as a means to produce various biologically active molecules. The technology is intended to result in the formation of pinhead sized cellulose-based porous capsules in which genetically modified live human cells can be encapsulated and maintained. In a laboratory setting, this proprietary live cell encapsulation technology has been shown to create a micro-environment in which encapsulated cells survive and flourish. They are protected from environmental challenges, such as the sheer forces associated with bioreactors and passage through catheters and needles, which we believe enables greater growth and production. The capsules are largely composed of cellulose (cotton) and are bio inert.
We are developing therapies for pancreatic and other solid cancerous tumors by using genetically engineered live human cells that we believe are capable of converting a cancer prodrug into its cancer-killing form. We encapsulate those cells using the Cell-in-a-Box® technology and place those capsules in the body as close as possible to the tumor. In this way, we believe that when a cancer prodrug is administered to a patient with a particular type of cancer that may be affected by the prodrug, the killing of the patient’s cancerous tumor may be optimized.
We have also been considering ways to exploit the benefits of the Cell-in-a-Box® technology to develop therapies for cancer that involve prodrugs based upon certain constituents of the Cannabis plant; these constituents are of the class of compounds known as “cannabinoids”.
Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; (ii) we validate our Cell-in-a-Box® encapsulation technology in our planned Phase 2b clinical trial in LAPC and (iii) the availability of sufficient additional funding, we are not spending any further resources developing this cannabinoid program.
In addition, we have been exploring ways to delay the production and accumulation of malignant ascites fluid that results from many types of abdominal cancerous tumors. Malignant ascites fluid is secreted by abdominal cancerous tumors into the abdomen after the tumors have reached a certain stage of growth. This fluid contains cancer cells that can seed and form new tumors throughout the abdomen. This fluid accumulates in the abdominal cavity, causing swelling of the abdomen, severe breathing difficulties and extreme pain.
| S-1 |
In our pancreatic cancer development program, our plan is to determine whether our product candidate can prevent or delay the production and accumulation of malignant ascites fluid. For the same reasons as those given above until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; (ii) we validate our Cell-in-a-Box® encapsulation technology in our planned Phase 2b clinical trial in LAPC and (iii) the availability of sufficient additional funding, we are not spending any further resources developing this malignant ascites fluid program.
We have also been developing a potential therapy for Type 1 diabetes and insulin-dependent Type 2 diabetes. Our product candidate for the treatment of diabetes consists of encapsulated genetically modified insulin-producing cells. The encapsulation will be done using the Cell-in-a-Box® technology. Implanting these cells in the body is designed to function as a bio-artificial pancreas for purposes of insulin production.
As with the two previous programs, until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND upon which the FDA has placed a clinical hold; (ii) we validate our Cell-in-a-Box® encapsulation technology in our planned Phase 2b clinical trial in LAPC and (iii) the availability of sufficient additional funding, we are not spending any further resources developing the diabetes program.
Cancer Therapy
Targeted Chemotherapy
Our live-cell encapsulation technology-based potential therapies consist of encapsulated genetically modified living cells, with the type of encapsulated cell dependent on the disease being treated. For our lead product candidate, a therapy for pancreatic cancer, we propose that approximately 15,000-20,000 genetically modified live cells that produce an enzyme (an isoform of cytochrome P450), which we believe will convert the chemotherapy prodrug ifosfamide into its cancer-killing form, will be encapsulated using the Cell-in-a-Box® technology. In the clinical trial, if the FDA allows us to proceed, approximately 300 of these capsules will be placed in the patients’ blood supply and guided into place using interventional radiography so that they finally reside as close to the tumor in the pancreas as possible. Low doses (one gram per square meter of body surface area of the patient) of the chemotherapy prodrug ifosfamide will then be given to the patient intravenously.
The prodrug ifosfamide is normally activated in the patient’s liver. By activating the prodrug near the tumor using the Cell-in-a-Box® capsules, we believe our cellular therapy will act as a type of “bio-artificial liver.” Using this type of “targeted chemotherapy,” we are seeking to create an environment that enables optimal concentrations of the “cancer-killing” form of ifosfamide at the site of the tumor. Because the cancer-killing form of ifosfamide has a short biological half-life, we believe that this approach will result in little to no collateral damage to other organs in the body. We also believe this treatment will significantly reduce tumor size with no treatment-related side effects.
| S-2 |
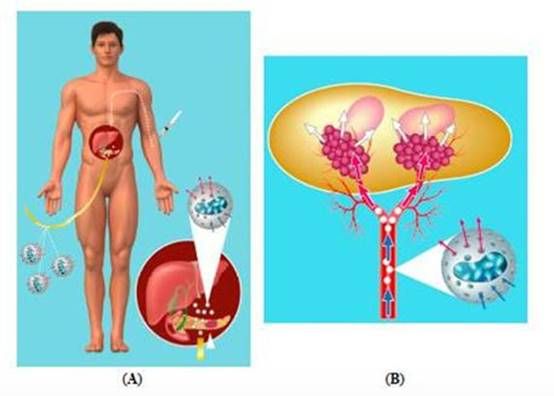
Figure 1: Proposed treatment for pancreatic cancer by targeted deployment and activation of chemotherapy using Cell-in-a-Box® encapsulated cells.
|
Note: Charts A and B are generalized graphic depictions of the principal hypothesized mechanisms of our proposed treatment for pancreatic cancer using our product candidate, the combination of Cell-in-a-Box® encapsulated cells plus low-doses of ifosfamide, under expected conditions. This combination therapy will be the subject of a clinical trial we plan to conduct, subject to FDA approval allowing us to move forward with our clinical trial. No regulatory authority has granted marketing approval for the Cell-in-a-Box® technology, the related encapsulated cells, or Cell-in-a-Box® and encapsulated cells plus low-dose ifosfamide combination. |
 |
|
Chart (A)
Cell-in-a-Box® capsules containing
live ifosfamide-activating cells (shown in white) will be implanted in the blood vessels leading to the tumor in the pancreas. Then low
dose ifosfamide will be given intravenously. |
Chart (B)
Chart B shows the human pancreas and generalized depictions of two pancreatic cancer tumors (shown in pink) as examples. In this chart, ifosfamide is converted to its cancer-killing form by the encapsulated live cells implanted near the tumors (shown in maroon).
Legend Blue Arrows: Ifosfamide enters capsules Red Arrows: Conversion to active form White Arrows: Activated ifosfamide targets tumors |
| S-3 |
Figure 2: Hypothesized mechanism of action of treatment for pancreatic cancer by targeted deployment of the encapsulated live cells and activation of the chemotherapy prodrug drug ifosfamide. The immune system cells are too large to enter the capsule.
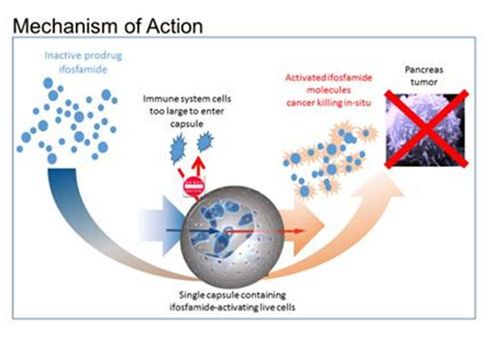
Pancreatic Cancer Therapy
We believe an unmet medical need exists for patients with LAPC whose pancreas tumor no longer responds after 4-6 months of treatment with either Abraxane® plus gemcitabine or the 4-drug combination known as FOLFIRINOX (folinic acid, fluorouracil, irinotecan and oxaliplatin). Both combinations are the current standards of care for pancreatic cancer. We believe that these refractory patients have no effective treatment alternative once their tumors no longer respond to these therapies. Two of the most commonly used treatments for these patients are 5-fluorouiracil (“5-FU”) or capecitabine (a prodrug of 5-FU) plus radiation (chemoradiation therapy). We believe that both treatments are only marginally effective in treating the tumor and both result in serious side effects. More recently, radiation treatment alone is being used at some cancer centers in the United States (“U.S.”).
Other treatments are being tried at various cancer centers in the U.S. in an attempt to address this lack of an effective treatment for many LAPC patients, but their success is far from certain. We are developing a therapy comprised of Cell-in-a-Box® encapsulated live cells implanted near the pancreas tumor followed by the infusion of low doses of the cancer prodrug ifosfamide. We believe that our therapy, if approved, can serve as a “consolidation therapy” that can be used with the current standards of care for LAPC and thus address this critical unmet medical need. Two previous human clinical trials of an encapsulated live cell and ifosfamide combination for LAPC were conducted in Germany by Bavarian Nordic during 1998 – 2000, and such trials were referenced in our IND for LAPC, submitted on September 1, 2020.
Subject to the FDA allowing us to move forward, we plan to commence a clinical trial involving patients with LAPC whose tumors have ceased to respond to either Abraxane® plus gemcitabine or FOLFIRINOX after 4-6 months of either therapy. The trial would initially take place in the U.S. with possible study sites in Europe at a later date.
| S-4 |
Our Investigational New Drug Application and the Clinical Hold
On September 1, 2020, we submitted an IND to the FDA for a planned Phase 2b clinical trial in LAPC. Shortly thereafter, we received Information Requests from the FDA related to the IND. We timely responded to all Information Requests.
On October 1, 2020, we received notice that the FDA had placed our IND on clinical hold.
On October 30, 2020, the FDA sent a letter to us setting forth the reasons for the clinical hold and providing specific guidance on what we must do to have the clinical hold lifted.
In order to address the clinical hold, the FDA has requested that we:
| · | Provide additional sequencing data and genetic stability studies; |
| · | Conduct a stability study on the final formulated drug product candidate as well as the cells from our Master Cell Bank; |
| · | Evaluate the compatibility of the delivery devices (the prefilled syringe and the microcatheter used to implant the CypCaps™) with our drug product candidate; |
| · | Provide additional detailed description of the manufacturing process; |
| · | Provide additional product release specifications for our encapsulated cells; |
| · | Demonstrate comparability between the 1st and 2nd generation products and ensure adequate and consistent product performance and safety between the two generations of product; |
| · | Conduct a biocompatibility assessment using the final finished capsules after the entire drug product candidate manufacturing process (but without cells); |
| · | Address insufficiencies in Chemistry, Manufacturing and Controls information in the cross-referenced Drug Master File; |
| · | Conduct an additional nonclinical study in a large animal (such as a pig) to assess the safety, activity and distribution of the drug product candidate; and |
| · | Revise the Investigators Brochure to include any additional preclinical studies conducted in response to the clinical hold and remove any statements not supported by the data. |
The FDA also requested that we address the following issues as an amendment to the IND:
| · | Provide a Certificate of Analysis for pc3/2B1 plasmid that includes tests for assessing purity, safety, and potency; |
| · | Perform qualification studies for the drug substance filling step to ensure that the product candidate remains sterile and stable during the filling process; |
| · | Submit an updated batch analysis for the drug product candidate for the specific lot that will be used for manufacturing all future drug product candidate; |
| · | Provide additional details for the methodology for the Resorufin (CYP2B1) potency and the PrestoBlue cell metabolic assays; |
| · | Provide a few examples of common microcatheters that fit the specifications in our Angiography Procedure Manual; |
| · | Clarify the language in the Pharmacy Manual regarding proper use of the syringe fill with the drug product candidate; and |
| · | Provide a discussion with data for trial of the potential for cellular and humoral immune reactivity against the heterologous rat CYP2B1 protein and potential for induction of autoimmune-mediated toxicities in our study population in the LAPC. |
| S-5 |
We have assembled a scientific and regulatory team of experts to address the FDA requests. That team is working to complete the items requested by the FDA. We are in varying stages of addressing the studies and acquiring the information requested by the FDA.
The following provides a summary of the activities in which we are engaged to have the clinical hold lifted:
| · | We have completed a 3, 6, 9, and 12-month product stability study of our clinical trial product (CypCaps™), including container closure integrity testing for certain timepoints; the next time point in this ongoing study will be at 18 months of product stability. |
| · | We have designed and commenced various additional studies required by the FDA. These include (i) a stability study on the cells from our Master Cell Bank (“MCB”) used to make the CypCaps™, which are already at the 3-year stability timepoint; (ii) further sequence analysis of the DNA encoding of the Cyp2B1 gene in the cells in the CypCaps™; and (iii) collated existing information on the reproducibility and quality of the filling of the MCB cells into vials ready for CypCaps™ manufacturing. |
| · | We have designed and commenced a Subchronic and Chronic Toxicity study. We have also designed and are awaiting initiation of biocompatibility studies, including: (i) a Skin Sensitization study; (ii) an Acute Systematic Toxicity study; (iii) an Ames test (Genotoxicity Bacteria and Reverse Mutation tests); (iv) an Intracutaneous test; (v) a Complement Activation test; (vi) a Hemolysis test; (vii) an In Vitro Cytotoxicity test; and (viii) an In Vivo Micronucleus assay. Some of the data being generated by these studies will also be used to demonstrate comparability with the CypCaps™ that were used in the two earlier German clinical trials over twenty years ago conducted by Bavarian Nordic. |
| · | To enable the biocompatibility studies to be performed, we had Austrianova manufacture and deliver an additional 400 syringes of empty capsules. |
| · | We designed and will initiate studies to show that CypCaps™ are not in any way adversely affected by the catheters used by interventional radiologists to deliver them, nor by the contract media used to visualize the blood vessels during implantation of the CypCaps™. |
| · | We designed and will initiate studies to demonstrate how robust the CypCaps™ are during delivery and use as well as to document that the syringes used to deliver the CypCaps™ will allow delivery consistently, smoothly and safely. |
| · | With our support, Austrianova is providing additional detailed confidential information to the FDA on the manufacturing process, including information on the improvements made to the live cell encapsulated product since the last clinical trials with respect to reproducibility and safety of the CypCaps™. |
| · | We plan to update our IND submission documents to include: (i) more pre-clinical data as discussed above, (ii) some additional parameters for release of the CypCaps™, (iii) a recommendation of the catheters and contrast medium to be used to deliver the CypCaps™; and (iv) an extensive discussion of the potential for cellular and humoral immune reactivity against the heterologous rat CYP2B1 protein and potential for induction of autoimmune-mediated toxicities in our study population in the LAPC. |
| · | We have designed an abbreviated study in pigs to address biocompatibility and long-term implantation of the capsules. This animal study will complement the positive data already available from the previous human clinical trials conducted by Bavarian Nordic showing the safety of CypCaps™ implantation for up to two years in humans. |
Cannabinoids to Treat Cancer
Numerous studies have demonstrated the therapeutic potential of certain cannabinoids (constituents of Cannabis) in patients with cancer. Two of the most widely studied cannabinoids in this regard are tetrahydrocannabinol (“THC”) and cannabidiol (“CBD”). Cannabinoids are potentially: (i) anti-proliferative (slow tumor growth); (ii) anti-metastatic (slow tumor spread); (iii) anti-angiogenic (slowing blood vessel development); and (iv) pro-apoptotic (initiate programmed cell death). In in vitro and in vivo models, the therapeutic potential of cannabinoids is broad. Results support the therapeutic potential in lung, brain, thyroid, lymphoma, liver, skin, pancreas, uterus breast and prostate cancers. In a review of 51 scientific studies, among other properties, it was observed that cannabinoids can regulate cellular signaling pathways critical for cell growth and survival. These properties indicate that cannabinoids could be useful in the treatment of cancer.
| S-6 |
We have many competitors that are developing Cannabis-based treatments for cancer. Jazz Pharmaceuticals has acquired GW Pharmaceuticals, PLC who had an approved cannabinoid product for the treatment of multiple sclerosis spasticity and was developing a product portfolio to treat a variety of illnesses, including glioblastoma (brain cancer). Cannabis Science, Inc. has been developing topical cannabinoid treatments for basal and squamous cell skin cancers and Kaposi’s sarcoma, and is exploring pre-clinical development of cannabinoid-based anti-cancer drugs in a collaborative agreement with other entities. OWC Pharmaceutical Research Corp. is developing Cannabis-based products targeting a variety of indications and has a collaborative agreement with an academic medical center in Israel to study the effects of cannabinoids on multiple myeloma (a cancer of plasma cells). Cannabis Pharmaceuticals, Inc. is developing personalized anti-cancer and palliative Cannabis-based treatments aimed mainly at improving the cachexia, anorexia syndrome and quality-of-life issues that are often characteristic of patients with devastating diseases like cancer.
In contrast to the work being done by these companies, we plan to focus on developing specific therapies based on chosen molecules rather than using complex Cannabis extracts. We intend to use the Cell-in-a-Box® technology in combination with genetically modified cell lines designed to activate cannabinoid molecules for the treatment of diseases and their related symptoms. Our initial target will be glioblastoma, a very difficult-to treat form of brain cancer.
In May 2014, we entered into a research agreement with the University of Northern Colorado (“UNC”). The goal of the original research was to develop methods for the identification, separation and quantification of constituents of Cannabis, some of which are prodrugs, which could potentially be used in combination with the Cell-in-a-Box® technology to treat cancer.
In January 2017, we entered into a second research agreement with UNC. The goal of this research is to assess the synthesis of the patG gene and its incorporation into a vector, transfection of human embryonic kidney cells using this vector and assessment of cannabinoic acid decarboxylase activity.
During 2017, UNC identified an organism whose genome contains the genetic code for production of an enzyme capable of activating a cannabinoid prodrug into its active cancer-killing form. Our Cannabis program now has two primary areas of focus. The first is evaluating the therapeutic potential of cannabinoids, such as THC and CBD, particularly in our main “target” tumor – glioblastoma. UNC’s laboratory research has confirmed that a purified cannabinoid showed a potent dose-dependent decrease in cell viability for various cancers, suggesting that this cannabinoid exhibits significant anti-proliferative effects (stops the growth and multiplication of cancer cells). This activity has been demonstrated in brain (glioblastoma), pancreas, breast, lung, colon and melanoma cancer cells. The second area of focus is in finding an enzyme capable of converting an inactive, side-effect-free, cannabinoid prodrug into its active cancer-killing form.
Clinically, targeted cannabinoid-based chemotherapy would be accomplished by implanting the encapsulated bio-engineered cells near the site of a tumor, along with administration of a cannabinoid prodrug which would become activated at the site of the tumor by an enzyme produced by the encapsulated cells. We believe this could lead to better efficacy than existing therapies with minimal treatment related adverse events.
Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; (ii) we validate our Cell-in-a-Box® encapsulation technology in our planned Phase 2b clinical trial in LAPC and (iii) the availability of sufficient additional funding occurs, we are not spending any further resources developing this program.
Malignant Ascites Fluid Therapy
We have been exploring ways to delay the production and accumulation of malignant ascites fluid that results from many types of abdominal tumors. Malignant ascites fluid is secreted by an abdominal tumor into the abdomen after the tumor reaches a certain stage of growth. This fluid contains cancer cells that can seed and form new tumors throughout the abdomen. As this ascites fluid accumulates in the abdominal cavity, it can cause gross swelling of the abdomen, severe breathing difficulties and extreme pain.
| S-7 |
Once an abdominal tumor reaches a certain stage of development, the tumor secretes malignant ascites fluid into the abdominal cavity. When that occurs, malignant ascites fluid must be removed by paracentesis on a periodic basis. This procedure is painful and costly. We know of no available therapy that prevents or delays the production and accumulation of malignant ascites fluid. Preclinical studies were conducted by Translational Drug Development (“TD2”), an early-stage Clinical Research Organization (“CRO”) specializing in oncology, to examine whether the combination of Cell-in-a-Box® encapsulated cells plus low doses of ifosfamide can delay the production and accumulation of malignant ascites fluid. We believe the data from these studies support our plans to further explore whether the treatment might play a role in malignant ascites fluid production and accumulation. However, the conclusions were difficult to interpret with certainty. As a result, we plan to conduct another preclinical study in Germany to determine if our conclusions from the TD2 studies are valid. If this study is successful, and subject to discussions with the FDA, we plan to submit an IND to seek approval from the FDA to conduct a Phase 1 clinical trial in the U.S. to determine if our drug product candidate can delay the production and accumulation of malignant ascites fluid.
Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; (ii) we validate our Cell-in-a-Box® encapsulation technology in our planned Phase 2b clinical trial in LAPC and (iii) the availability of sufficient additional funding occurs, we are not spending any further resources developing this program.
Diabetes Therapy
A Bio-Artificial Pancreas to Treat Diabetes
We are developing a therapy for Type 1 diabetes and insulin-dependent Type 2 diabetes based upon the encapsulation of a human liver cell line genetically engineered to produce, store and secrete insulin at levels in proportion to the levels of blood sugar in the human body. We are also considering an alternative route to bringing a biological treatment for diabetes into the clinic. We are exploring the possibility of encapsulating human insulin-producing stem cells and then transplanting them into a diabetic patient. Our plans are subject to discussions with the FDA.
The cell line we select will be encapsulated using the Cell-in-a-Box® encapsulation technology. If appropriate animal testing is completed successfully, and subject to discussions with the FDA, we intend to submit an IND to seek the FDA’s approval to transplant encapsulated insulin-producing cells into diabetic patients. The goal for these approaches is to develop a bio-artificial pancreas for purposes of insulin production for diabetics who are insulin-dependent.
Our diabetes program began with two of the most critical components of a biological diabetes therapy - a line of human cells which release insulin in response to the blood glucose level in their environment and a technology to protect the cells from an attack by the immune system once they are transplanted into a patient’s body to replace his or her own destroyed insulin-producing cells. This technology is the Cell-in-a-Box® encapsulation technology. The cells used are called Melligen cells. They are patent-protected and have been licensed to us by University of Technology Sydney (“UTS”).
Regulations for the use of living cells as a medical product require that the potential of the cells to grow and form a tumor in a patient be assessed. This so-called “tumorigenicity study” has been completed by the University of Veterinary Medicine Vienna (“VetMed”). Melligen cells showed very low tumorigenicity at a level we believe would expect to pass regulatory scrutiny, although this is subject to discussions with the FDA.
Putting Melligen cells and the Cell-in-a-Box® technology together, we conducted the first functional study in diabetic mice. The results did not meet our expectations. We discovered that, contrary to what we had expected and what we had read in published scientific papers on the Melligen cells published by UTS, the cells are not stable. With extensive testing and experiments, we discovered that the Melligen cells lose some of their specific beneficial properties over time.
We entered into a new research agreement with UTS to create an advanced version of the Melligen cells for the treatment of diabetes. Under the new research agreement, improvements will be made to the Melligen cells that we believe will increase their stability, increase their insulin production and increase the bioactivity of the produced insulin.
| S-8 |
Prof. Ann Simpson, who created the Melligen cells, and her team of research scientists at UTS have been conducting this research project. The work is being funded by the Company and UTS. Our portion of the funding was previously paid to UTS. The research to date has not produced the results we had anticipated and is taking longer than we anticipated. It remains to be seen whether the Melligen cells are capable of producing the required insulin to be a viable cell line for the treatment of diabetes.
Until: (i) the FDA allows us to commence a clinical trial in LAPC described in our IND for which the FDA has placed a clinical hold; (ii) we validate our Cell-in-a-Box® encapsulation technology in our planned Phase 2b clinical trial in LAPC and (iii) the availability of sufficient additional funding occurs, we are not spending any further resources developing this program.
Impact of the COVID-19 Pandemic on the Company’s Operations
The coronavirus SARS-Cov2 pandemic (“COVID-19”) is causing significant, industry-wide delays in clinical trials. Although we are not yet in a clinical trial, we have filed an IND with the FDA to commence a clinical trial in LAPC. While the IND has been placed on clinical hold by the FDA, we have assessed the impact of COVID-19 on our operations. As of the date of this prospectus supplement, we believe the COVID-19 pandemic has had an impact upon our operations, primarily relating to delays in tasks associated with the preparation of the Company’s responses to the FDA’s clinical hold, including all requested preclinical studies and assays. There may be further delays in generating responses to the requests from the FDA related to the clinical hold. Many of these delays are due to the impact of the COVID-19 pandemic in foreign countries where we are conducting these preclinical studies and assays, including India, Europe, Singapore and Thailand. There have also been supply chain interruptions due to the COVID-19 pandemic.
Further, many clinical trials have been delayed due to COVID-19. There are numerous reasons for these delays. For example, patients have shown a reluctance to enroll or continue in a clinical trial due to fear of exposure to COVID-19 when they are in a hospital or doctor’s office. There are local, regional and state-wide orders and regulations restricting usual normal activity by people. These discourage and interfere with patient visits to a doctor’s office if the visit is not COVID-19 related. Healthcare providers and health systems have shifted their resources away from clinical trials toward the care of COVID-19 patients. The FDA and other healthcare providers are making product candidates for the treatment of COVID-19 a priority over product candidates unrelated to COVID-19.
As a result of COVID-19 and the mitigation efforts to address it, we may experience additional disruptions that could adversely impact our business and clinical trial, including: (i) delays or difficulties in enrolling patients in our Phase 2b clinical trial if the FDA allows us to go forward with the trial; (ii) delays or difficulties in clinical site activation, including difficulties in recruiting clinical site investigators and clinical site personnel; (iii) delays in clinical sites receiving the supplies and materials needed to conduct our clinical trial, including interruption in global shipping that may affect the transport of our clinical trial product; (iv) changes in local regulations as part of a response to the COVID-19 pandemic which may require us to change the ways in which our clinical trial is to be conducted, which may result in unexpected costs, or to discontinue the clinical trial altogether; (v) diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trial; (vi) interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others, or interruption of clinical trial subject visits and study procedures, the occurrence of which could affect the integrity of clinical trial data; (vii) risk that participants enrolled in our clinical trials will acquire COVID-19 while the clinical trial is ongoing, which could impact the results of the clinical trial, including by increasing the number of observed adverse events; (viii) delays in necessary interactions with local regulators, ethics committees, and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; (ix) limitations in employee resources that would otherwise be focused on the conduct of our clinical trial because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; (x) refusal of the FDA to accept data from clinical trials in affected geographies; and (xi) interruption or delays to our clinical trial activities.
As a result of the COVID-19 pandemic, commencement of our planned clinical trial to treat LAPC may be delayed beyond the lifting of the clinical hold by the FDA should that occur. Also, enrollment may be difficult for the reasons discussed above. In addition, after enrollment in the trial, if patients contract COVID-19 during their participation in the trial or are subject to isolation or shelter in place restrictions, this may cause them to drop out of our clinical trial, miss scheduled therapy appointments or follow-up visits or otherwise fail to follow the clinical trial protocol. If patients are unable to follow the clinical trial protocol or if the trial results are otherwise affected by the consequences of the COVID-19 pandemic on patient participation or actions taken to mitigate COVID-19 spread, the integrity of data from the clinical trial may be compromised or not be accepted by the FDA. This could further adversely impact or delay our clinical development program if the FDA allows it to proceed.
| S-9 |
It is highly speculative in projecting the effects of COVID-19 on our proposed clinical development program and the Company generally. Moreover, the various precautionary measures taken by many governmental authorities around the world in order to limit the spread of COVID-19 has had and may continue to have an adverse effect on the global markets and global economy, including on the availability and pricing of employees, resources, materials, manufacturing and delivery efforts and other aspects of the global economy. The continuation of the COVID-19 pandemic could materially disrupt our business and operations, hamper our ability to raise additional funds or sell or securities, continue to slow down the overall economy, curtail consumer spending, interrupt our sources of supply, and make it hard to adequately staff our operations. The effects of COVID-19 quickly and dramatically change over time. Its evolution is difficult to predict, and no one is able to say with certainty when the pandemic will cease to have an impact on our operations.
Recent Developments
Certificate of Amendment to Articles of Incorporation
On June 30, 2021, at our Annual Meeting of Stockholders, our stockholders approved a Certificate of Amendment to our Articles of Incorporation to increase the number of authorized shares of common stock from 2,500,000,000 shares to 50,000,000,000 shares. Upon effectiveness of the 1:1,500 reverse stock split on July 12, 2021, the number of authorized shares of our common stock was reduced proportionately to 33,333,334 shares by operation of Nevada law and the number of outstanding shares of our common stock was reduced to 1,591,420 shares.
Reverse Stock Split
On June 30, 2021, our Board of Directors (“Board”) approved a reverse stock split of 1:1,500 of our authorized and our issued and outstanding shares of common stock effective on July 12, 2021 pursuant to a Certificate of Change filed in Nevada. Except as otherwise indicated, all share and per share information in this prospectus supplement gives effect to the reverse stock split of the Company’s outstanding common stock, which was effected at a ratio of 1-for-1,500 shares as of 12:01 a.m. Eastern Time on Monday, July 12, 2021.
Preliminary Fiscal Year 2021
On August 2, 2021, the Company announced selected preliminary unaudited financial results for the fiscal year ended April 30, 2021. Complete audited financial information and operating data for the fiscal year ended April 30, 2021, will not be available until after this offering is complete.
Preliminary (unaudited) Fiscal 2021 Financial Results
Net loss for the fiscal year ended April 30, 2021 was approximately $3.6 million, or approximately $2.45 basic and diluted loss per share, compared with a net loss of approximately $3.8 million, or approximately $4.23 basic and diluted loss per share, for the fiscal year ended April 30, 2020. The losses per share amounts are based on the basic weighted average number of shares outstanding.
Cash on Hand
As of April 30, 2021, we had approximately $2.2 million in cash in our bank account.
The preliminary fiscal year ended April 30, 2021 financial data is unaudited, preliminary, based upon the Company’s good faith estimates and the information and data currently available and subject to completion of the Company's financial closing procedures. While the Company expects that its final financial results for the fiscal year ended April 30, 2021, following the completion of its financial closing procedures, will generally be consistent with the amounts provided herein, the Company's actual results may differ materially from these estimates as a result of the completion of its financial closing procedures, as well as final adjustments and other developments that may arise between now and the time that its financial results for the fiscal year ended April 30, 2021 are finalized.
Summary of Risks Associated with Our Business
Our business is subject to numerous risks and uncertainties that you should consider before investing in our company. These risks are described more fully in the section titled “Risk Factors” in this prospectus supplement. These risks include, but are not limited to, the following:
| · | We are a biotechnology company with limited resources, a limited operating history and have no products approved for clinical trials or commercial sale, which may make it difficult to evaluate our current business and predict our future success and viability. |
| · | As a result of the clinical hold that has been placed on our IND by the FDA, it has taken and may continue to take considerable time and expense to respond to the FDA and no assurance can be given that the FDA will remove the clinical hold in which case our business and prospects will likely suffer material adverse consequences. |
| · | The recent and ongoing COVID-19 pandemic has affected and could continue to affect our operations, as well as the business or operations of third parties with whom we conduct business. Our business could be adversely affected by the effects of other future health pandemics in regions where we or third parties on which we rely have significant business operations. |
| · | If we are unable to successfully raise additional capital, our future clinical trials and product development could be limited and our long-term viability may be threatened. |
| S-10 |
| · | Due to the significant resources required for the development of our programs, and depending on our ability to access capital, we must prioritize development of certain product candidates. We may expend our limited resources on programs that do not yield a successful product candidate and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success. |
| · | We currently have no commercial revenue and may never become profitable. |
| · | Our ability to continue as a going concern. |
| · | If we are unable to obtain, or if there are delays in obtaining, required approval from the applicable regulatory agencies, we will not be able to commercialize our product candidates and our ability to generate revenue will be materially impaired. |
| · | If allowed to proceed with our clinical development program, we intend to conduct clinical trials for certain of our product candidates at sites outside of the U.S., and the U.S. regulatory agencies may not accept data from trials conducted in such locations. |
| · | Promising results in previous clinical trials of our encapsulated live cell and ifosfamide combination for LAPC may not be replicated in future clinical trials which could result in development delays or a failure to obtain marketing approval. |
| · | We may not be able to protect our intellectual property rights throughout the world. |
| · | We rely and expect to continue to rely heavily on third parties to conduct our preclinical studies and clinical trials, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such studies and trials. |
| · | There are risks related to this offering, including risks that you will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase in this offering and the risk that substantial future sales or other issuances of our common stock could depress the market for our common stock. |
| · | We have broad discretion in the use of the net proceeds of this offering and, despite our efforts, we may use the net proceeds in a manner that does not increase the value of your investment. |
| · | There has been no consistent active trading market for our common stock, and public trading of our common stock may continue to fluctuate substantially. |
| · | A large number of shares may be issued and subsequently sold upon the exercise of existing options and warrants. |
| · | We are a “smaller reporting company” under the SEC’s disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies. |
| · | As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act. |
| S-11 |
| · | As a result of our approval to be listed on Nasdaq concurrent with this offering, we will incur materially increased costs and become subject to additional regulations and requirements. |
| · | There can be no assurance that we will be able to comply with the continued listing standards of Nasdaq, including the ability to continue to comply with the minimum bid price requirement of Nasdaq, a failure of which could result in a de-listing of our common stock, limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions. |
| · | Following the reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve. |
Our Corporate Information
We are a Nevada corporation incorporated in 1996. In 2013, we restructured our operations to focus on biotechnology. The restructuring resulted in the Company focusing all of its efforts upon the development of a novel, effective and safe way to treat cancer and diabetes. In January 2015, the Company changed its name from Nuvilex, Inc. to PharmaCyte Biotech, Inc. to reflect the nature of its current business.
Our corporate headquarters is located at 23046 Avenida de la Carlota, Suite 600, Laguna Hills, California 92653, and our telephone number is (917) 595-2850. We maintain a website at www.pharmacyte.com, to which we regularly post copies of our press releases as well as additional information about us. Our filings with the SEC will be available free of charge through the website as soon as reasonably practicable after being electronically filed with or furnished to the SEC. Information contained in our website is not a part of, nor incorporated by reference into, this prospectus or our other filings with the SEC, and should not be relied upon.
| S-12 |
| Common stock offered by us | shares of common stock. |
| Pre-funded Warrants offered by us | Pre-funded Warrants to purchase up to an aggregate of shares of common stock. We are also offering to each purchaser the opportunity to purchase, if the purchaser so chooses, Pre-funded Warrants, in lieu of shares of common stock. Each Pre-funded Warrant will be exercisable for one share of our common stock. The purchase price of each Pre-funded Warrant will equal the price per share at which the shares of common stock are being sold to the public in this offering, minus $0.001, and the exercise price of each Pre-funded Warrant will be $0.001 per share. This offering also relates to the shares of common stock issuable upon exercise of any Pre-funded Warrants sold in this offering. The exercise price and number of shares of common stock issuable upon exercise will be subject to certain further adjustments as described herein. See “Description of Securities Offered” on page S-45 of this prospectus supplement. |
| Common Warrants offered by us | Common Warrants to purchase an aggregate of shares of our common stock. Each share of our common stock and each Pre-funded Warrant to purchase one share of our common stock is being sold together with a Common Warrant to purchase one share of our common stock. Each Common Warrant has an exercise price of $ per share, is immediately exercisable and will expire on the anniversary of the original issuance date. The shares of common stock or the Pre-funded Warrants, and the accompanying Common Warrants, as the case may be, can only be purchased together in this offering but will be issued separately and will be immediately separable upon issuance. This offering also relates to the offering of the shares of common stock issuable upon exercise of the Common Warrants. See “Description of Securities Offered” on page S-45 of this prospectus supplement. |
| Underwriter’s option to purchase additional shares and/or Common Warrants | We have granted the underwriter an option for a period of up to 30 days from the date of this prospectus supplement to purchase up to an additional shares of our common stock and/or Common Warrants to purchase additional shares of our common stock at the public offering price less the underwriting discounts and commissions. |
| Common stock to be outstanding after this offering | shares ( shares assuming the underwriter exercises in full its option to purchase additional shares and/or Common Warrants), assuming all of the Pre-funded Warrants issued in this offering are exercised and no exercise of any Common Warrants issued in this offering. |
| Use of proceeds | We intend to use the net proceeds of this offering (i) to complete activities requested by the FDA in order to address the FDA’s clinical hold on our IND with respect to our planned Phase 2b clinical trial in LAPC, including conducting several additional preclinical studies and assays and providing the FDA with the additional information it requested, (ii) to begin to fund and conduct the Phase 2b clinical trial in LAPC, if and when the clinical hold on the IND is lifted, and (iii) for general working capital purposes. See “Use of Proceeds.” |
| Risk factors | An investment in our common stock involves a high degree of risk. See “Risk Factors” beginning on page S-15 of this prospectus supplement, and under similar headings in other documents filed after the date hereof and incorporated by reference into this prospectus supplement and the accompanying prospectus. |
| S-13 |
| Underwriter’s Warrants | We will issue to the underwriter, or its designees, at the closing of this offering common stock purchase warrants to purchase the number of shares of common stock equal to 7.5% of the aggregate number of shares of common stock and Pre-funded Warrants sold in this offering, including shares sold upon the underwriter’s exercise of its option to purchase additional shares. The underwriter’s warrants will be exercisable immediately and will expire three years from the issuance date and are registered on the registration statement of which this prospectus is a part. The exercise price of the underwriter’s warrants will equal 125% of the public offering price per share and the accompanying warrant in this offering. See “Underwriting.” |
| OTCQB ® Market Symbol | The Company’s OTCQB Market Symbol is PMCB, although as a result of the recent reverse split of the Company’s common stock, until August 6, 2021, the Company’s OTCQB Market Symbol has been PMCBD. Our common stock will continue to trade on the OTCQB Venture Market until trading commences on Nasdaq. There is no established trading market for the Pre-funded Warrants or the Common Warrants, and we do not expect a trading market to develop. We do not intend to list the Pre-funded Warrants or Common Warrants on any securities exchange or nationally recognized trading system. Without a trading market, the liquidity of the Pre-funded Warrants and the Common Warrants will be extremely limited. |
| Anticipated Nasdaq Symbol | Our common stock has been approved for listing on the Nasdaq Capital Market (“Nasdaq”) under the symbol “PMCB.” |
| Reverse Stock Split | On June 30, 2021, our Board approved a reverse stock split of 1:1,500 of our authorized and our issued and outstanding shares of common stock effective on July 12, 2021. Unless otherwise stated, all share and per share information in this prospectus supplement reflects the reverse stock split of the authorized and outstanding common stock of the Company at a ratio of 1:1,500. However, share and per share amounts in the accompanying prospectus and certain of the documents incorporated by reference herein have not been adjusted to give effect to the reverse stock split. |
Outstanding Shares
The number of shares of our common stock to be outstanding after this offering is based on 1,591,420 shares of our common stock outstanding as of July 31, 2021 (subject to adjustment based on issuances of additional shares as applicable due to the rounding up on fractional shares resulting from the 1:1,500 reverse stock split), and excludes:
| · | 42,333 shares reserved for issuance upon the exercise of outstanding stock options at a weighted average exercise price of $78.63 per share; |
| · | 2,981 shares reserved for issuance upon the exercise of outstanding warrants at a weighted average exercise price of $58.70 per share; |
| · | 166,667 shares of common stock reserved for future issuance under our 2021 Equity Incentive Plan; |
| · | shares of common stock issuable upon exercise of the Common Warrants issued in this offering; and |
| · | up to shares of common stock (or if the underwriter exercises its option to purchase additional shares of common stock in full, up to shares of common stock) issuable upon exercise of warrants with an exercise price of $ per share to be issued to the underwriter or its designees as compensation in connection with this offering. |
Except as otherwise indicated, all information in this prospectus supplement assumes (i) no exercise by the underwriter of its option to purchase additional shares of our common stock and/or Common Warrants; (ii) no exercise or conversion of the outstanding options or warrants described above; (iii) no exercise of the Pre-funded Warrants and (iv) no exercise of the Common Warrants offered and sold in this offering.
| S-14 |
Investing in our common stock involves a high degree of risk. Before investing in our common stock, you should carefully consider the risks described below, together with all of the other information contained in this prospectus supplement and the accompanying prospectus and incorporated by reference herein and therein, including from our Annual Report on Form 10-K for the fiscal year ended April 30, 2020, our Quarterly Reports on Form 10-Q for the periods ending October 31, 2020 and January 31, 2021 as well as any amendment or update to our risk factors reflected in subsequent filings with the SEC. Some of these factors relate principally to our business and the industry in which we operate. Other factors relate principally to your investment in our securities. The risks and uncertainties described therein and below are not the only risks facing us. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also materially and adversely affect our business and operations.
If any of the matters included in the following risks were to occur, our business, financial condition, results of operations, cash flows or prospects could be materially and adversely affected. In such case, you may lose all or part of your investment.
Risks Related to Our Financial Position, FDA Clinical Hold, Need for Additional Capital and Overall Business
We are a biotechnology company with limited resources, a limited operating history and have no products approved for clinical trials or commercial sale, which may make it difficult to evaluate our current business and predict our future success and viability.
We are a biotechnology company focused on developing cellular therapies for cancer based upon a proprietary cellulose-based live cell encapsulation technology known as “Cell-in-a-Box®.” In recent years, we have devoted substantially all our resources to the development of our product candidate for LAPC. We have limited resources, a limited operating history, no products approved for clinical trials or commercial sale and therefore have not produced any revenues. We have generated significant operating losses since our inception. Our net losses for the years ended April 30, 2020 and 2019 were approximately $3.8 million and $4.1 million, respectively. As of April 30, 2020, we had an accumulated deficit of approximately $104 million. As of January 31, 2021, we had an accumulated deficit of $106 million, and we reported a net loss of $2.6 million for the nine months ended January 31, 2021. Substantially all our losses have resulted from expenses incurred relating to our research and development programs and from general and administrative expenses and operating losses associated with our business.
We expect to continue to incur significant expenses and operating losses for the foreseeable future. We anticipate these losses will increase as we continue our research and development of, and, if approved by the FDA, commence clinical trials for, our product candidates. In addition to budgeted expenses, we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business.
We have no facilities to conduct fundamental research and we have performed our research and development activities by collaboration with contract service providers and contract manufacturers, and by designing and developing research programs in collaboration with university-based experts who work with us to evaluate mechanism(s) of disease for which we have designed and developed product candidates. We have not maintained a principal laboratory or primary research facility for the development of our product candidates.
Biotechnology product development is a highly uncertain undertaking and involves a substantial degree of risk. We have not commenced or completed clinical trials for any of our product candidates, obtained marketing approval for any product candidates, manufactured a commercial scale product, or arranged for a third party to do so on our behalf, or conducted sales and marketing activities necessary for successful product commercialization. Given the highly uncertain nature of biotechnology product development, we may never commence or complete clinical trials for any of our product candidates, obtain marketing approval for any product candidates, manufacture a commercial scale product or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful product commercialization.
Our limited operating history as a company makes any assessment of our future success and viability subject to significant uncertainty. We will encounter risks and difficulties frequently experienced by early-stage biotechnology companies in rapidly evolving fields, and we have not yet demonstrated an ability to successfully overcome such risks and difficulties. If we do not address these risks and difficulties successfully, our business, operating results and financial condition will suffer.
| S-15 |
As a result of the clinical hold that has been placed on our IND by the FDA, it has taken and may continue to take considerable time and expense to respond to the FDA and no assurance can be given that the FDA will remove the clinical hold in which case our business and prospects will likely suffer material adverse consequences.
On October 1, 2020, we received notice from the FDA that it had placed our IND for a planned Phase 2b clinical trial in LAPC on clinical hold. As part of the clinical hold process, the FDA has asked for additional information, tasks to be performed by us and new preclinical studies and assays. It has taken and may continue to take a considerable period of time, the length of which is not certain at this time, for us to conduct such tasks and preclinical studies and to generate and prepare the requested information. In addition, the significant expense of such work is likely to require us to raise additional capital. It is possible that the service providers that we will utilize for such work may have considerable backlogs and/or are suffering from slowdowns as a result of COVID-19 and may not be able to perform such work for an extended period of time. Even if we are able to fully respond to the FDA’s requests, they may subsequently make additional requests that we would need to fulfill prior to the lifting of the clinical hold and we may never be able to begin our clinical trial in LAPC, obtain regulatory approval or successfully commercialize our product candidates. An inability to conduct our clinical trial in LAPC as a result of the clinical hold or otherwise, would likely force us to terminate our clinical development plans. It is possible that we will be unable to fully respond to the FDA in a satisfactory manner, and as a result the clinical hold may never be lifted. If the clinical hold is not lifted or if the lifting takes an extended period of time, our business and prospects will likely suffer material adverse consequences.
The recent and ongoing COVID-19 pandemic could materially affect our operations, as well as the business or operations of third parties with whom we conduct business. Our business could be adversely affected by the effects of other future health pandemics in regions where we or third parties on which we rely have significant business operations.
Our business and its operations, including, but not limited to, our proposed clinical development program, supply chain operations, research and development activities and fundraising activities, has been and could continue to be adversely affected by the COVID-19 pandemic in areas where we have business operations, including the U.S., India, Europe, Singapore and Thailand. Also, this pandemic could cause significant disruption in the operations of third parties upon whom we rely on to conduct the Company’s business. In March 2020, the World Health Organization declared the COVID-19 outbreak a pandemic. Shortly thereafter, the U.S. government-imposed restrictions on travel between the U.S., Europe, and certain other countries. The President of the U.S. declared the COVID-19 pandemic a national emergency. Since March 2020, numerous state, regional and local jurisdictions, including the jurisdictions where our headquarters are located, as well as foreign jurisdictions, have imposed, and others in the future may impose, quarantines, shelter-in-place orders, executive, and similar government orders for their residents to control the spread of COVID-19. The COVID-19 pandemic has had an impact upon our operations.
The effects of the executive orders, the shelter-in-place orders and our work-from-home policies has and may continue to negatively impact productivity, disrupt our business, and delay our proposed clinical development program and timeline, the magnitude of which will depend, in part, on the length and severity of the restrictions and other limitations on our ability to conduct our business in the ordinary course. These and similar, and perhaps more severe, disruptions in our operations could negatively impact our business, operating results and financial condition.
Quarantines, shelter-in-place, executive, and similar government orders, or the perception that such orders, shutdowns or other restrictions on the conduct of business operations could occur, related to COVID-19, could impact personnel at our third-party manufacturing facilities in Thailand, or the availability or cost of materials we use or require to conduct our business, including product development, which would disrupt our supply chain. Some of our suppliers and vendors of certain materials used in our operations and research and development activities are located in areas that are subject to executive orders and shelter-in-place orders. While many of these materials may be obtained from more than one supplier, port closures and other restrictions resulting from the COVID-19 pandemic may disrupt our supply chain or limit our ability to obtain sufficient materials to operate our business. To date, we are aware of certain suppliers for our research and development activities that have experienced operational delays directly related to the COVID-19 pandemic.
| S-16 |
Depending upon the length of the COVID-19 pandemic and whether the FDA lifts the clinical hold on our IND, we anticipate our planned clinical trial in LAPC may be affected by the COVID-19 pandemic. If COVID-19 continues to spread in the U.S. and elsewhere, we may experience additional disruptions that could adversely impact our business and proposed clinical trial, including: (i) delays or difficulties in enrolling patients in our Phase 2b clinical trial if the FDA allows us to go forward with such trial; (ii) delays or difficulties in clinical site activation, including difficulties in recruiting clinical site investigators and clinical site personnel; (iii) delays in clinical sites receiving the supplies and materials needed to conduct our clinical trial, including interruption in global shipping that may affect the transport of our clinical trial product; (iv) changes in local regulations as part of a response to the COVID-19 pandemic which may require us to change the ways in which our clinical trial is to be conducted, which may result in unexpected costs, or to discontinue the clinical trial altogether, if allowed to proceed; (v) diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as our clinical trial sites and hospital staff supporting the conduct of our clinical trial; (vi) interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by federal or state governments, employers and others, or interruption of clinical trial subject visits and study procedures, the occurrence of which could affect the integrity of clinical trial data; (vii) risk that participants enrolled in our proposed clinical trials will acquire COVID-19 while the clinical trial is ongoing, which could impact the results of the clinical trial, including by increasing the number of observed adverse events; (viii) delays in necessary interactions with local regulators, ethics committees, and other important agencies and contractors due to limitations in employee resources or forced furlough of government employees; (ix) limitations in employee resources that would otherwise be focused on the conduct of our clinical trial because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; (x) refusal of the FDA to accept data from clinical trials in affected geographies; and (xi) interruption or delays to our clinical trial activities.
The spread of COVID-19, which has caused a widespread impact throughout the world, may materially affect us economically. The potential economic impact brought about by the COVID-19 pandemic, and the duration of such impact, is difficult to assess or predict. The pandemic has resulted in significant disruption of global financial markets, which could reduce our ability to access capital and negatively affect our future liquidity. Also, a recession or market correction resulting from the spread of COVID-19 and related government orders and restrictions could materially affect our business and the value of our common stock. The COVID-19 pandemic continues to evolve. The ultimate impact of the COVID-19 pandemic and the mitigation efforts to address it is highly uncertain and subject to change. We do not yet know the full extent of potential delays or impacts on our business, our proposed clinical trial, healthcare systems or the global economy.
If we are unable to successfully raise additional capital, our future clinical trials and product development could be limited and our long-term viability may be threatened.
We have experienced negative operating cash flows since our inception and have funded our operations primarily through sales of our equity securities. We will need to seek additional funds in the future through equity or debt financings, or strategic alliances with third parties, either alone or in combination with equity financings to complete our product development initiatives. These financings could result in substantial dilution to the holders of our common stock, or require contractual or other restrictions on our operations or on alternatives that may be available to us. If we raise additional funds by issuing debt securities, these debt securities could impose significant restrictions on our operations. Any such required financing may not be available in amounts or on terms acceptable to us, and the failure to procure such required financing could have a material and adverse effect on our business, financial condition and results of operations, or threaten our ability to continue as a going concern.
Our operating and capital requirements during this fiscal year and thereafter will vary based on several factors, including whether the FDA allows us to commence our planned clinical trial for LAPC, how quickly enrollment of patients in our such trial can be commenced, the duration of the clinical trial and any change in the clinical development plans for our product candidates and the outcome, timing and cost of meeting regulatory requirements established by the FDA and the EMA or other comparable foreign regulatory authorities. The proceeds of this proposed offering will not be sufficient to complete our planned Phase 2b clinical trial for LAPC if the clinical hold is lifted by the FDA.
| S-17 |
Our present and future capital requirements will be significant and will depend on many factors, including:
| · | whether the FDA lifts the clinical hold on our IND filing for LAPC; |
| · | the progress and results of our development efforts for our product candidates; |
| · | the costs, timing and outcome of regulatory review of our product candidates; |
| · | the costs and timing of preparing, filing and prosecuting patent applications, maintaining and enforcing our intellectual property rights and defending any intellectual property-related claims; |
| · | the effect of competing technological and market developments; |
| · | market acceptance of our product candidates; |
| · | the rate of progress in establishing coverage and reimbursement arrangements with domestic and international commercial third-party payors and government payors; |
| · | the extent to which we acquire or in-license other products and technologies; and |
| · | legal, accounting, insurance and other professional and business-related costs. |
We may not be able to acquire additional funds on acceptable terms, or at all. If we are unable to raise adequate funds, we may have to liquidate some or all of our assets, or delay or reduce the scope of or eliminate some or all of our development programs. Further, if we do not have, or are not able to obtain, sufficient funds, we may be required to delay development or commercialization of our product candidates. We also may have to reduce the resources devoted to our product candidates or cease operations. Any of these factors could harm our operating results.
Due to the significant resources required for the development of our programs, and depending on our ability to access capital, we must prioritize development of certain product candidates. We may expend our limited resources on programs that do not yield a successful product candidate and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
We seek to maintain a process of prioritization and resource allocation to maintain an optimal balance between aggressively advancing lead programs and ensuring replenishment of our portfolio. Until such time, if ever, as the FDA lifts its clinical hold on our IND related to our planned Phase 2b clinical trial in LAPC, our Cell-in-a-Box® encapsulation technology is validated in our planned Phase 2b clinical trial, and sufficient additional funding is available, we have halted spending on behalf of our other development programs with respect to cannabinoids, malignant ascites fluid and diabetes.
Due to the significant resources required for the development of our programs, we must focus our programs on specific diseases and decide which product candidates to pursue and advance and the amount of resources to allocate to each. Our decisions concerning the allocation of research, development, collaboration, management and financial resources toward particular product candidates or therapeutic areas may not lead to the development of any viable commercial product and may divert resources away from better opportunities. Similarly, our potential decisions to delay, terminate or collaborate with third parties in respect of certain programs may subsequently also prove to be suboptimal and could cause us to miss valuable opportunities. We may fail to capitalize on viable commercial products or profitable market opportunities, be required to forego or delay pursuit of opportunities with other product candidates or other diseases that may later prove to have greater commercial potential than those we choose to pursue, or relinquish valuable rights to such product candidates through collaboration, licensing or other royalty arrangements in cases in which it would have been advantageous for us to invest additional resources to retain sole development and commercialization rights. If we make incorrect determinations regarding the viability or market potential of any or all of our programs or product candidates or misread trends in the biotechnology industry, our business, prospects, financial condition and results of operations could be materially adversely affected.
| S-18 |
We currently have no commercial revenue and may never become profitable.
Even if we can successfully achieve regulatory approval for our product candidates, we do not know what the reimbursement status of our product candidates will be or when any of these products will generate revenue for us, if at all. We have not generated, and do not expect to generate, any product revenue for the foreseeable future. We expect to continue to incur significant operating losses for the foreseeable future due to the cost of our research and development, preclinical studies and clinical trials and the regulatory approval process for our product candidates. The amount of future losses is uncertain and will depend, in part, on the rate of growth of our expenses.
Our ability to generate revenue from our product candidates also depends on numerous additional factors, including our ability to:
| · | successfully complete development activities, including the remaining preclinical studies and planned clinical trials for our product candidates; |
| · | complete and submit NDAs or BLAs to the FDA and MAAs to the EMA, and obtain regulatory approval for indications for which there is a commercial market; |
| · | complete and submit applications to, and obtain regulatory approval from, other foreign regulatory authorities; |
| · | manufacture any approved products in commercial quantities and on commercially reasonable terms; |
| · | develop a commercial organization, or find suitable partners, to market, sell and distribute approved products in the markets in which we have retained commercialization rights; |
| · | achieve acceptance among patients, clinicians and advocacy groups for any products we develop; |
| · | obtain coverage and adequate reimbursement from third parties, including government payors; and |
| · | set a commercially viable price for any products for which we may receive approval. |
We are unable to predict the timing or amount of increased expenses, or when or if we will be able to achieve or maintain profitability. Even if we can complete the processes described above, we anticipate incurring significant costs associated with commercializing our product candidates.
We face substantial competition, which may result in others discovering, developing or commercializing competing products before or more successfully than we do.
The development and commercialization of new drug products is highly competitive. We face competition with respect to our current product candidates. We will face competition with respect to any product candidates that we may seek to develop or commercialize in the future. Such competition may arise from major pharmaceutical companies, specialty pharmaceutical companies and biotechnology companies worldwide. There are several large pharmaceutical and biotechnology companies that currently market products or are pursuing the development of products for the treatment of the disease indications for which we are developing our product candidates. Some of these competitive products and therapies are based on scientific approaches that are entirely different from our approach. Potential competitors also include academic institutions, government agencies and other public and private research organizations that conduct research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and commercialization.
Specifically, there are numerous companies developing or marketing therapies for cancer and diabetes, including many major pharmaceutical and biotechnology companies. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we can enter the market.
| S-19 |
Many of the companies against which we are competing or against which we may compete in the future have significantly greater financial resources and expertise in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals and marketing approved products than we do. Mergers and acquisitions in the pharmaceutical and biotechnology sectors may result in even more resources being concentrated among a smaller number of our competitors. Smaller and other early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These third parties compete with us in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs.
Additional Risks Related to Regulatory Matters
If we are unable to obtain, or if there are delays in obtaining, required approval from the applicable regulatory agencies, we will not be able to commercialize our product candidates and our ability to generate revenue will be materially impaired.
Our product candidates must obtain marketing approval from the FDA for commercialization in the U.S. and from foreign regulatory agencies for commercialization in countries outside the U.S. The process of obtaining marketing approvals in the countries in which we intend to sell and distribute our product candidates is expensive and can take many years, if approval is obtained at all. This process can vary substantially based upon a variety of factors, including the type, complexity and novelty of the product candidates involved. Failure to obtain marketing approval for a product candidate will prevent us from commercializing that product candidate. To date, we have not received approval to market any of our product candidates from regulatory agencies in any jurisdiction. We have no experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third-party contract research organizations to assist us in this process. Securing marketing approval requires the submission of extensive preclinical and clinical data and supporting information to the regulatory agencies for each product candidate to establish the product candidate’s safety and efficacy. Securing marketing approval also requires the submission of information about the product manufacturing process to, and inspection of manufacturing facilities by, the regulatory agencies.
Our product candidates may not be effective, may be only moderately effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use. Regulatory agencies have substantial discretion in the approval process and may refuse to accept any application or may decide that our data are insufficient for approval and require additional preclinical, clinical or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit or prevent marketing approval of a product candidate. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may also cause delays in or prevent the approval of an application. New cancer drugs frequently are indicated only for patient populations that have not responded to an existing therapy or have relapsed after such therapies. If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for our product candidates may be harmed and our ability to generate revenues will be materially impaired.
If allowed to proceed with our clinical development programs, we intend to conduct clinical trials for certain of our product candidates at sites outside of the U.S., and the U.S. regulatory agencies may not accept data from trials conducted in such locations.
Although the FDA may accept data from clinical trials conducted outside the U.S., acceptance of this data is subject to certain conditions imposed by the regulatory agencies outside of the U.S. For example, the clinical trial must be well designed and conducted and performed by qualified investigators in accordance with ethical principles. The trial population must also adequately represent the population in the country in which the clinical trial is being conducted. The data must be applicable to the U.S. population and medical practice in the U.S. in ways that the FDA deems clinically meaningful. Generally, the patient population for any clinical trial conducted outside of the U.S. must be representative of the population for whom we intend to seek approval in the U.S.
In addition, while these clinical trials are subject to the applicable local laws, the FDA acceptance of the data will be dependent upon its determination that the trials also complied with all applicable U.S. laws and regulations. There can be no assurance that the FDA will accept data from trials conducted outside of the U.S. If the FDA does not accept the data from any of our clinical trials that we determine to conduct outside the U.S., it would likely result in the need for additional trials that would be costly and time-consuming and delay or permanently halt the development of our product candidate.
| S-20 |
In addition, the conduct of clinical trials outside the U.S. could have a significant impact on us. Risks inherent in conducting international clinical trials include:
| · | Foreign regulatory requirements that could restrict or limit our ability to conduct our clinical trials; |
| · | Administrative burdens of conducting clinical trials under multiple foreign regulatory schemes; |
| · | Foreign exchange fluctuations; and |
| · | Diminished protection of intellectual property in some countries. |
Promising results in previous clinical trials of our encapsulated live cell and ifosfamide combination for LAPC may not be replicated in future clinical trials which could result in development delays or a failure to obtain marketing approval.
Positive results in the previous Phase 1/2 and Phase 2 clinical trials of the encapsulated live cell and ifosfamide combination product may not be predictive of similar results in future clinical trials such as our planned Phase 2b clinical trial in LAPC for which the FDA has placed a clinical hold. The previous Phase 1/2 and Phase 2 clinical trials were done over twenty years ago in Germany by Bavarian Nordic and had a relatively limited number of patients in each trial. These trials resulted in outcomes that were not statistically significant and may not be representative of future results. In addition, interim results obtained after a clinical trial has commenced do not necessarily predict results in future clinical trials. Numerous companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in early-stage clinical development. Our clinical trials may produce negative or inconclusive results and we may decide, or regulatory agencies may require us, to conduct additional clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain the approval for their products by the regulatory agencies.
Risks related to our Intellectual Property
We may not be able to protect our intellectual property rights throughout the world.
Filing, prosecuting and defending patents or establishing other intellectual property rights to our product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States or non-existent. For example, the Melligen cells are protected by patents only in the U.S. and Europe and we are only pursuing patent protection for our pancreatic cancer product candidate in the U.S., Australia and Canada.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of some countries do not favor the enforcement of patents and other intellectual property protection, which could make it difficult for us to stop the infringement of our patents or misappropriation of our intellectual property rights generally. Proceedings to enforce our patent and other intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents or intellectual property rights at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful.
Many countries, including European Union countries, India, Japan and China, have compulsory licensing laws under which a patent owner may be compelled under specified circumstances to grant licenses to third parties. In those countries, we may have limited remedies if patents are infringed or if we are compelled to grant a license to a third party, which could materially diminish the value of those patents. This could limit our ability to pursue strategic alternatives, including identifying and consummating transactions with potential third-party partners, to further develop, obtain marketing approval for and/or commercialize our product candidates, and consequently our potential revenue opportunities.
| S-21 |
Our intellectual property and data and market exclusivity may not be sufficient to block others from commercializing identical or competing products.
Our success depends in large part on our ability to obtain and maintain both intellectual property rights and data and market exclusivity for our product candidates in order to block others from commercializing identical or competing products. Establishing intellectual property rights includes filing, prosecuting, maintaining, and enforcing patents that cover our product candidates and variations of our product candidates and protecting our trade secrets and other proprietary information related to our product candidates from unauthorized use.
The foundational patents relating to the Cell-in-the-Box® technology that were formerly licensed from Bavarian Nordic/GSF covering capsules encapsulating cells expressing cytochrome P450 and treatment methods using the same expired on March 27, 2017. Currently, we do not have any issued patents in any countries covering our product candidate for the treatment of pancreatic cancer. We exclusively license from UTS patented Melligen cells, which cover our product candidate for the treatment of diabetes, which are issued in the U.S. and Europe and expire in August 2028. Currently, we do not have any issued patents or pending applications covering our product candidate for the treatment of cancer using cannabinoids or our product candidate for the treatment of malignant ascites fluid therapy. We may not be able to obtain protection for our product candidates or variations of our product candidates. Even if our owned and licensed patent applications issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors from competing with us or otherwise provide us with any competitive advantage or our patents may expire before or shortly after our product candidate is approved. Our competitors may be able to circumvent our owned or licensed patents by developing similar or alternative technologies or products in a non-infringing manner.
Confidential know-how and trade secrets are only protectable to the extent a third party utilizes the confidential know-how or trade secret in an unauthorized manner; however, if a third party is able to independently duplicate the technology, such as through reverse engineering, without access to or use of our confidential know-how or trade secret, we would have no recourse.
In addition, data exclusivity that is provided through the BPCIA in the U.S. and equivalents in foreign countries is limited in both time and scope. The BPCIA bars the FDA from approving biosimilar applications for 12 years after an innovator biological product receives initial marketing approval, however it does not bar the FDA from approving an identical or similar product that is the subject of its own BLA. Finally, upon the approval of the first BLA for a biologic designated as an Orphan Drug for a specified indication, the sponsor of that BLA is entitled to 7 years of exclusive marketing rights in the U.S. for biologic for the particular indication unless the sponsor cannot assure the availability of sufficient quantities to meet the needs of persons with the disease. In Europe, this exclusivity is 10 years. However, Orphan Drug status for an approved indication does not prevent another company from seeking approval of a biologic that has other labeled indications that are not under orphan or other exclusivities. In addition, in the U.S., the FDA is not prevented from approving another biologic for the same labeled Orphan indication if the company can demonstrate that the other biologic is clinically superior to first approved product.
Even if we are able to obtain patents and maintain confidential information and trade secrets and obtain data and market exclusivity for our product candidates, our competitors may be able to develop and obtain approval of identical or competing products.
If we do not obtain patent and/or data exclusivity for our product candidates, our business may be materially harmed.
Our commercial success will largely depend on our ability to obtain and maintain patent and other intellectual property protection and/or data exclusivity under the BPCIA in the U.S. and other countries with respect to our proprietary technology, product candidates and our target indications. If we are unable to obtain patents covering our product candidates or obtain data and/or marketing exclusivity for our product candidates, our competitors may be able to take advantage of our investment in development and clinical trials by referencing our clinical and preclinical data to obtain approval of competing products, such as a biosimilar, earlier than might otherwise be the case.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for some of our technology and product candidates, we also rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain our competitive position. We seek to protect these trade secrets, in part, by entering non-disclosure and confidentiality agreements with parties who have access to them, such as our employees, corporate collaborators, outside scientific collaborators, contract manufacturers, consultants, advisors and other third parties. We seek to protect our confidential proprietary information, in part, by entering confidentiality and invention or patent assignment agreements with our employees and consultants; however, we cannot be certain that such agreements have been entered with all relevant parties.
| S-22 |
Moreover, to the extent we enter such agreements, any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets to unaffiliated third parties. We may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming and the outcome is unpredictable. In addition, some courts inside and outside the U.S. are less willing or unwilling to protect trade secrets. If any of our trade secrets were to be lawfully obtained or independently developed by a competitor, we would have no right to prevent them, or those to whom they communicate them, from using that technology or information to compete with us. If any of our trade secrets were to be disclosed to or independently developed by a competitor, our competitive position would be harmed.
The majority of the technology that we license and use for our product candidates is not protected by patents, but rather is based upon confidential know-how and trade secrets. Confidential know-how and trade secrets are only protectable to the extent a third party utilizes the confidential know-how or trade secret in an unauthorized manner; however, if a third party is able to independently duplicate the technology, such as through reverse engineering, without access to or use of our confidential know-how or trade secret, we would have no recourse.
Risks Related to Our Dependence on Third Parties
We rely heavily on third parties to conduct our preclinical studies and plan to rely on third parties to conduct our clinical trials, assuming they are allowed to proceed, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such studies and trials.
We currently rely heavily on third parties to conduct our preclinical studies and plan to rely on third parties to conduct our clinical trials, assuming they are allowed to proceed, including Austrianova in which we own an equity interest. We expect to continue to rely heavily on third parties, such as a CRO, a clinical data management organization, a medical institution, a clinical investigator and others to plan for and conduct our clinical trials. Our agreements with these third parties generally allow the third party to terminate our agreement with them at any time. If we are required to enter alternative arrangements because of any such termination, the introduction of our product candidates to market could be delayed.
Our reliance on these third parties for research and development (“R&D”) activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we design our clinical trials and will remain responsible for ensuring that each is conducted in accordance with the general investigational plan and protocol for the trial. Moreover, regulatory agencies require us to comply with current good manufacturing practices (“cGMP”) for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity and confidentiality of trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. We also are required to register ongoing clinical trials and post the results of completed clinical trials on a government-sponsored database of regulatory agencies within specified timeframes. Failure to do so can result in fines, adverse publicity and civil and criminal sanctions.
Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. If these third parties do not successfully carry out their contractual duties, meet expected deadlines or conduct our clinical trials in accordance with the requirements of a regulatory agency or our protocols, we will not be able to obtain, or may be delayed in obtaining, marketing approvals for our product candidates and will not be able to, or may be delayed in our efforts to, successfully commercialize our product candidates.
We expect to rely on third parties to store and distribute our product candidates for our clinical trials. Any performance failure on the part of such third parties could delay clinical development or marketing approval of our product candidates or commercialization of our products, producing additional losses and depriving us of potential product candidate revenue. Our existing collaboration with universities and institutions is important to our business. If we are unable to maintain these collaborations, or if these collaborations are not successful, our business could be adversely affected.
We rely on numerous consultants for a substantial portion of our R&D related to our product candidates. If there are delays or failures to perform their obligations, our product candidates would be adversely affected. If our collaboration with these consultants is unsuccessful or is terminated, we would need to identify new research and collaboration partners for our preclinical and clinical development. If we are unsuccessful or significantly delayed in identifying new collaboration and research partners, or unable to reach an agreement with such a partner on commercially reasonable terms, development of our product candidates will suffer, and our business would be materially harmed.
| S-23 |
Furthermore, if any of these consultants change their strategic focus, or if external factors cause any one of them to divert resources from our collaboration, or if any one of them independently develops products that compete directly or indirectly with our product candidates using resources or information it acquires from our collaboration, our business and results of operations could suffer.
We rely on Prof. Günzburg, Dr. Salmons and Dr. Löhr for the development of our product candidates. If they decide to terminate their relationship with us, we may not be successful in the development of our product candidates.
We rely on Prof. Walter H. Günzburg and Dr. Brian Salmons, officers of Austrianova, and Dr. Löhr, currently with the Karolinska Institute in Stockholm, Sweden, for the development of our product candidates. If they decide to terminate their relationship with us, we may not be successful in the development of our product candidates.
Prof. Günzburg, Dr. Salmons and Dr. Löhr are involved in almost all our scientific endeavors underway and being planned by us. These endeavors include preclinical and clinical studies involving our cancer therapy for LAPC to be conducted in the U.S. and elsewhere on our behalf. They also provide professional consulting services to us through the respective consulting agreements we have entered with the consulting companies through which they provide services. The consulting agreements may be terminated for any reason at any time upon one party giving the other a written notice prior to the effective date of the termination. If that occurs, we may not be successful in the development of our product candidates which could have a material adverse effect on us.
Risks Related to this Offering
You will experience immediate and substantial dilution in the net tangible book value per share of the common stock you purchase in this offering.
The effective public offering price per share in this offering may exceed the net tangible book value per share of our common stock outstanding prior to this offering, in which case you may incur an immediate and substantial dilution in the net tangible book value of the shares of common stock you purchase in this offering or the shares of common stock underlying the Pre-funded Warrants and the Common Warrants you purchase in this offering. After giving effect to the sale by us of (i) shares of our common stock and accompanying Common Warrants to purchase shares of our common stock at the public offering price of $ per share of common stock and accompanying Common Warrant, and (ii) Pre-funded Warrants to purchase shares of common stock and accompanying Common Warrants at an effective public offering price of $ per Pre-funded Warrant and accompanying Common Warrant, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us and assuming full exercise of the Pre-funded Warrants, you will experience immediate dilution of $ per share, representing the difference between the effective public offering price per share and our as adjusted net tangible book value per share as of January 31, 2021 after giving effect to this offering. The exercise of warrants, including the Common Warrants issued in this offering, exercise of outstanding stock options and vesting of other stock awards may result in further dilution of your investment. See “Dilution” on page S-34 of this prospectus supplement for a more detailed discussion of the dilution you will incur if you participate in this offering.
In addition, as of July 31, 2021, we had outstanding options to acquire 42,333 shares of our common stock and outstanding warrants to acquire 2,981 shares of our common stock, and pursuant to this offering, we will issue underwriter warrants to purchase up to shares of our common stock. The issuance of shares of our common stock upon exercise of the stock options or warrants could result in dilution to the interests of other holders of our common stock and could adversely affect our stock price.
Substantial future sales or other issuances of our common stock could depress the market for our common stock.
Sales of a substantial number of shares of our common stock, or the perception by the market that those sales could occur, could cause the market price of our common stock to decline or could make it more difficult for us to raise funds through the sale of equity in the future.
| S-24 |
In connection with this offering, our directors and executive officers have entered into lock-up agreements for a period of 90 days following this offering. Our directors and executive officers may be released from such lock-up agreements prior to the expiration of the lock-up period at the sole discretion of H.C. Wainwright & Co. (See “Underwriting” beginning on page S-40 of this prospectus supplement). Upon expiration or earlier release of the lock-up, our directors and executive officers may sell shares into the market, which could adversely affect the market price of shares of our common stock.
Future issuances of our common stock or our other equity securities could further depress the market for our common stock. We expect to continue costs associated with our R&D programs, such as preclinical studies, clinical trials, and the regulatory approval process for therapeutic candidates, and general and administrative costs associated with our operations, and to satisfy our funding requirements, we may need to sell additional equity securities. The proceeds of this offering will not be sufficient to conduct our planned clinical trial in LAPC should the FDA lift its clinical hold on our IND and the Company may have to sell additional equity securities to fully fund such trial. The sale or the proposed sale of substantial amounts of our common stock or our other equity securities may adversely affect the market price of our common stock and our stock price may decline substantially. Our stockholders may experience substantial dilution and a reduction in the price that they are able to obtain upon sale of their shares. New equity securities issued may have greater rights, preferences or privileges than our existing common stock.
There is no public market for the Pre-funded Warrants or Common Warrants being offered in this offering.
There is no established public trading market for the Pre-funded Warrants or Common Warrants being offered in this offering, and we do not expect a market to develop. In addition, we do not intend to apply to list the Pre-funded Warrants or Common Warrants on any securities exchange or nationally recognized trading system, including Nasdaq. Without an active market, the liquidity of the Pre-funded Warrants and the Common Warrants will be limited.
The Common Warrants being offered may not have value.
The Common Warrants being offered by us in this offering have an exercise price of $ per share, subject to certain adjustments, and expire from the date of issuance, after which date any unexercised Common Warrants will expire and have no further value. In the event that the market price of our common stock does not exceed the exercise price of the Common Warrants during the period when they are exercisable, the Common Warrants may not have any value.
Holders of Pre-funded Warrants and Common Warrants purchased in this offering will have no rights as common stockholders until such holders exercise their Pre-funded Warrants or Common Warrants and acquire our common stock.
Until holders of Pre-funded Warrants or Common Warrants acquire shares of our common stock upon exercise of such warrants, holders of Pre-funded Warrants and Common Warrants will have no rights with respect to the shares of our common stock underlying such Pre-funded Warrants and Common Warrants. Upon exercise of the Pre-funded Warrants and Common Warrants, the holders will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
We have broad discretion in the use of the net proceeds of this offering and, despite our efforts, we may use the net proceeds in a manner that does not increase the value of your investment.
Our management will have broad discretion in the application of our existing cash and the net proceeds from this offering, including for any of the purposes described in the section titled "Use of Proceeds," and you will not have the opportunity as part of your investment decision to assess whether such proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of our existing cash and the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our existing cash and the net proceeds from this offering in ways that ultimately increase the value of your investment. The failure by our management to apply these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
| S-25 |
There has been no consistent active trading market for our common stock, and public trading of our common stock may continue to fluctuate substantially.
There has never been a consistent active trading market for our common stock and trading has been limited and sporadic. The Company’s OTCQB Market Symbol is PMCB, although as a result of the recent reverse split of the Company’s common stock, until August 6, 2021, the Company’s OTCQB Market Symbol has temporarily been “PMCBD.” Although our common stock sold in this offering has been approved for listing on Nasdaq under the symbol “PMCB” it has not yet commenced trading.
There is no assurance that the trading market for our common stock will become more active or liquid. Furthermore, there can be no assurance any market maker will be interested in trading our stock. Therefore, it may be difficult to sell your shares of common stock if you desire or need to sell them. As a result, no assurances can be given that you will be able to readily sell your common stock at a price equal to or above the price you paid. Our underwriter is not obligated to make a market in our securities, and even if they make a market, they can discontinue market making at any time without notice. Neither we nor the underwriter can provide any assurance that an active and liquid trading market in our securities will develop or, if developed, that such market will continue.
Moreover, the trading price of our common stock has fluctuated substantially over the past few years, and there remains a significant risk that our common stock price may continue to fluctuate substantially in the future in response to various factors, including any material developments in the FDA approval process for our proposed Phase 2b clinical trial, material variations in our periodic operating results, departures or additions of management or other key personnel, announcements of acquisitions, mergers, share consolidations, or new technology or patents, new product developments, significant litigation matters, gain or loss of significant licensees, significant capital transactions, substantial sales of our common stock in our trading market, and general and specific market and economic conditions.
We may not be able to meet the continued listing requirements for the Nasdaq Capital Market or another nationally recognized stock exchange, which could limit investors’ ability to make transactions in our securities and subject us to additional trading restrictions.
Our common stock has been approved for listing on Nasdaq under the symbol “PMCB” but has not yet commenced trading.
If and when our common stock is listed on the Nasdaq Capital Market, in order to remain listed on the Nasdaq Capital Market, we will be required to meet the continued listing requirements of the Nasdaq Capital Market or any other U.S. or nationally recognized stock exchange to which we may apply and be approved for listing. We may be unable to satisfy these continued listing requirements, and there is no guarantee that our common stock will remain listed on the Nasdaq Capital Market or any other U.S. or nationally recognized stock exchange. If, after listing, our common stock is delisted from the Nasdaq Capital Market or any other U.S. or nationally recognized stock exchange, we could face significant material adverse consequences, including:
| · | a limited availability of market quotations for our common stock; |
| · | reduced liquidity with respect to the market for our common stock; |
| · | a determination that our common stock is a “penny stock,” which will require brokers trading in our common stock to adhere to different rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our common stock; |
| · | a limited amount of news and analyst coverage; and |
| · | decreased ability to issue additional shares of our common stock or obtain additional financing in the future. |
| S-26 |
A large number of shares may be issued and subsequently sold upon the exercise of existing options and warrants.
As of July 31, 2021, there were 42,333 shares of common stock issuable under outstanding options and 2,981 shares issuable upon exercise of outstanding warrants at various exercise prices. To the extent that holders of existing options or warrants sell the shares of common stock issued upon the exercise of warrants, the market price of our common stock may decrease due to the additional selling pressure in the market. The risk of dilution from issuances of shares of common stock underlying existing options and warrants may cause shareholders to sell their common stock, which could further decline in the market price. In addition, we will be issuing in this offering Common Warrants to purchase shares of common stock which are immediately exercisable.
You may experience future dilution as a result of future equity offerings.
In order to raise additional capital, we may in the future offer additional common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing shareholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering.
We are a “smaller reporting company” under the SEC’s disclosure rules and have elected to comply with the reduced disclosure requirements applicable to smaller reporting companies.
We are a “smaller reporting company” under the SEC’s disclosure rules, meaning that we have either:
| · | a public float of less than $250 million; or |
| · | annual revenues of less than $100 million during the most recently completed fiscal year; and |
| · | no public float; or |
| · | a public float of less than $700 million. |
As a smaller reporting company, we are permitted to comply with scaled-back disclosure obligations in our SEC filings compared to other issuers, including with respect to disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We have elected to adopt the accommodations available to smaller reporting companies. Until we cease to be a smaller reporting company, the scaled-back disclosure in our SEC filings will result in less information about our company being available than for other public companies.
If investors consider our common stock less attractive as a result of our election to use the scaled-back disclosure permitted for smaller reporting companies, there may be a less active trading market for our common stock and our share price may be more volatile.
As a non-accelerated filer, we are not required to comply with the auditor attestation requirements of the Sarbanes-Oxley Act.
We are a non-accelerated filer under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and we are not required to comply with the auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act of 2002. Therefore, our internal controls over financial reporting will not receive the level of review provided by the process relating to the auditor attestation included in annual reports of issuers that are subject to the auditor attestation requirements. In addition, we cannot predict if investors will find our common stock less attractive because we are not required to comply with the auditor attestation requirements. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and trading price for our common stock may be negatively affected.
| S-27 |
If and when our common stock is listed on Nasdaq, we will incur materially increased costs and become subject to additional regulations and requirements.
If and when our common stock is listed on the Nasdaq Capital Market, as a newly exchange-listed public company, we will incur material additional legal, accounting and other expenses, including payment of annual exchange fees, to satisfy the continued listing standards for Nasdaq. If our common stock is listed on Nasdaq, we must meet certain financial and liquidity criteria to maintain our listing. If we fail to meet any of Nasdaq’s listing standards, our common stock may be delisted. In addition, our Board may determine that the cost of maintaining our listing on a national securities exchange outweighs the benefits of such listing. A delisting of our common stock from Nasdaq may materially impair our stockholders’ ability to buy and sell our common stock and could have an adverse effect on the market price of, and the efficiency of the trading market for, our common stock. The delisting of our common stock could significantly impair our ability to raise capital and the value of your investment.
We may experience volatility in our stock price, which may adversely affect the trading price of our common stock.
We have experienced significant volatility from time to time in the market price of our shares of common stock. Over the past twelve months, shares of our common stock were quoted and traded at a high of $55.50 per share and a low of $5.56 per share. In the future, the market price of our common stock may continue to be volatile.
Risks Related to our Reverse Stock Split
We cannot assure you that we will be able to continue to comply with the minimum bid price requirement of Nasdaq.
There can be no assurance that the market price of our common stock will remain at the level required for continuing compliance with Nasdaq’s minimum bid requirement. It is not uncommon for the market price of a company’s common stock to decline in the period following a reverse stock split. If the market price of our common stock declines following the effectuation of the reverse stock split, the percentage decline may be greater than would occur in the absence of a reverse stock split. In any event, other factors unrelated to the number of shares of our common stock outstanding, such as negative financial or operational results, could adversely affect the market price of our common stock and jeopardize our ability to maintain compliance with Nasdaq’s minimum bid price requirement.
There can be no assurance that we will be able to comply with the continued listing standards of Nasdaq, a failure of which could result in a de-listing of our common stock.
Nasdaq requires that the trading price of its listed stocks remain above one dollar in order for the stock to remain listed. If a listed stock trades below one dollar for more than 30 consecutive trading days, then it is subject to delisting from Nasdaq. In addition, to maintain a listing on Nasdaq, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity and certain corporate governance requirements. If we are unable to satisfy these requirements or standards, we could be subject to delisting. This would have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a delisting, we can provide no assurance that any action we may take to restore our compliance with the listing requirements would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the minimum bid price requirement, or prevent future non-compliance with the listing requirements.
The reverse stock split may decrease the liquidity of the shares of our common stock.
The liquidity of the shares of our common stock may be affected adversely by the reverse stock split given the reduced number of shares that will be outstanding following the reverse stock split, especially if the market price of our common stock does not increase as a result of the reverse stock split. In addition, the reverse stock split may increase the number of shareholders who own odd lots (less than 100 shares) of our common stock, creating the potential for such shareholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales.
| S-28 |
Following the reverse stock split, the resulting market price of our common stock may not attract new investors, including institutional investors, and may not satisfy the investing requirements of those investors. Consequently, the trading liquidity of our common stock may not improve.
Although we believe that a higher market price of our common stock may help generate greater or broader investor interest, there can be no assurance that the reverse stock split will result in a share price that will attract new investors, including institutional investors. In addition, there can be no assurance that the market price of our common stock will satisfy the investing requirements of those investors. As a result, the trading liquidity of our common stock may not necessarily improve.
Risks Related to our Tax Matters
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
As of April 30, 2020, we had federal net operating loss carryforwards of approximately $45 million, which will begin to expire in varying amounts beginning in 2020. Under Sections 382 and 383 of the United States Internal Revenue Code of 1986, as amended, or the Code, and corresponding provisions of state law, if a corporation undergoes an “ownership change” (generally defined as a greater than 50-percentage-point cumulative change (by value) in the equity ownership of certain stockholders over a rolling three-year period), the corporation’s ability to use its pre-change net operating loss carryforwards and other pre-change tax attributes to offset its post-change taxable income or taxes may be limited. We may have experienced ownership changes in the past and could experience one or more ownership changes in the future, including in connection with this offering, some of which are outside our control. Our net operating loss carryforwards may also be subject to limitation under state laws. Further, our ability to utilize net operating loss carryforwards of companies that we may acquire in the future may also be subject to limitations. There is also a risk that due to tax law changes, such as suspensions on the use of net operating loss carryforwards, or other unforeseen reasons, our ability to use our pre-change net operating loss carryforwards and other pre-change tax attributes to offset post-change taxable income or taxes may be subject to limitation or expire.
Changes in U.S. tax law could adversely affect our business and financial condition.
The laws, rules and regulations dealing with U.S. federal, state, and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect us or holders of our common stock. In recent years, many changes have been made to applicable tax laws and changes are likely to continue to occur in the future.
For example, the Tax Cuts and Jobs Act, or the TCJA, was enacted in 2017 and made significant changes to corporate taxation, including the reduction of the corporate tax rate from a top marginal rate of 35% to a flat rate of 21%, the limitation of the tax deduction for net interest expense to 30% of adjusted taxable income (except for certain small businesses), the limitation of the deduction for net operating losses from taxable years beginning after December 31, 2017 to 80% of current year taxable income and the elimination of net operating loss carrybacks generated in taxable years ending after December 31, 2017 (though any such net operating losses may be carried forward indefinitely), and the modification or repeal of many business deductions and credits. In addition, on March 27, 2020, then President Trump signed into law the “Coronavirus Aid, Relief, and Economic Security Act” or the CARES Act, which, among other things, suspends the 80% limitation on the deduction for net operating losses arising in taxable years beginning before January 1, 2021, permits a five-year carryback of net operating losses arising in taxable years beginning after December 31, 2017 and before January 1, 2021, and generally modifies the limitation on the deduction for net interest expense to 50% of adjusted taxable income for taxable years beginning in 2019 and 2020.
It cannot be predicted whether, when, in what form, or with what effective dates, new tax laws may be enacted, or regulations and rulings may be enacted, promulgated or issued under existing or new tax laws, which could result in an increase in our or our shareholders’ tax liability or require changes in the manner in which we operate in order to minimize or mitigate any adverse effects of changes in tax law or in the interpretation thereof.
| S-29 |
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement contains forward-looking statements. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. Forward-looking statements involve risks and uncertainties and include statements regarding, among other things, our projected revenue growth and profitability, our growth strategies and opportunity, anticipated trends in our market and our anticipated needs for working capital. They are generally identifiable by use of the words “may,” “will,” “should,” “anticipate,” “estimate,” “plans,” “potential,” “projects,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend” or the negative of these words or other variations on these words or comparable terminology. In particular, these include statements relating to future actions, prospective products, market acceptance, future performance or results of current and anticipated products, sales efforts, expenses, and the outcome of contingencies such as legal proceedings and financial results.
Examples of forward-looking statements in this prospectus supplement include, but are not limited to, our expectations regarding our business strategy, business prospects, operating results, operating expenses, working capital, liquidity and capital expenditure requirements. Important assumptions relating to the forward-looking statements include, among others, assumptions regarding demand for our products, the cost, terms and availability of materials related to biopharma products, pricing levels, the timing and cost of capital expenditures, status of regulatory approvals, competitive conditions and general economic conditions. These statements are based on our management’s expectations, beliefs and assumptions concerning future events affecting us, which in turn are based on currently available information. These assumptions could prove inaccurate. Although we believe that the estimates and projections reflected in the forward-looking statements are reasonable, our expectations may prove to be incorrect.
Important factors that could cause actual results to differ materially from the results and events anticipated or implied by such forward-looking statements include, but are not limited to:
| · | our ability to conduct the preclinical studies and assays and provide the additional information requested by the FDA in order to lift the clinical hold on our IND for LAPC; |
| · | our ability to advance any product candidates into, and successfully complete, clinical studies and obtain regulatory approval for them; |
| · | the timing or likelihood of regulatory filings and approvals; |
| · | the commercialization, marketing and manufacturing of our product candidates, if approved; |
| · | our ability to continue as a going concern; |
| · | the pricing and reimbursement of our product candidates, if approved; |
| · | the rate and degree of market acceptance and clinical utility of any products for which we receive marketing approval; |
| · | the implementation of our strategic plans for our business, product candidates and technology; |
| · | the scope of protection we have and are able to establish and maintain for intellectual property rights covering our product candidates and technology; |
| · | our expectations related to the use of proceeds from this offering and our existing cash resources, and estimates of our expenses, future revenues, capital requirements and our needs for additional financing; |
| S-30 |
| · | our ability to maintain and establish collaborations; |
| · | our financial performance; |
| · | our ability to maintain compliance with the Nasdaq Capital Market’s listing standards; |
| · | developments relating to our competitors and our industry, including the impact of government regulation; |
| · | our ability to retain and attract senior management and consultants; and |
| · | other risks and uncertainties, including those listed under the caption “Risk Factors” in this prospectus supplement and the accompanying prospectus or any other documents incorporated by reference in this prospectus supplement and the accompanying prospectus. |
We operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for us to predict all of those risks, nor can we assess the impact of all of those risks on our business or the extent to which any factor may cause actual results to differ materially from those contained in any forward-looking statement. The forward-looking statements in this prospectus supplement are based on assumptions management believes are reasonable. However, due to the uncertainties associated with forward-looking statements, you should not place undue reliance on any forward-looking statements. Further, forward-looking statements speak only as of the date they are made, and unless required by law, we expressly disclaim any obligation or undertaking to publicly update any of them in light of new information, future events, or otherwise.
| S-31 |
We estimate that the net proceeds from our issuance and sale of shares of our common stock, Pre-funded Warrants and Common Warrants in this offering will be approximately $ million, or approximately $ million if the underwriter exercises its option to purchase additional shares and/or Common Warrants to purchase additional shares in full, after deducting the estimated underwriting discounts and commissions and the estimated offering expenses payable by us. These estimates exclude the proceeds, if any, from the exercise of the Pre-funded Warrants and Common Warrants sold in this offering.
We intend to use the net proceeds of this offering (i) to complete activities requested by the FDA in order to address the FDA’s clinical hold on our IND with respect to our planned Phase 2b clinical trial in LAPC, including conducting several additional preclinical studies and assays and providing the FDA with the additional information it requested, (ii) to begin to fund and conduct the Phase 2b clinical trial in LAPC, if and when the clinical hold on the IND is lifted and (iii) for general corporate purposes.
The amounts and timing of our use of the net proceeds from this offering will depend on a number of factors, such as the timing and progress of our efforts to lift the FDA’s clinical hold on our IND for a clinical trial for LAPC, our ability to conduct the clinical trial for LAPC if and when the FDA’s clinical hold is lifted, our R&D efforts, the timing and progress of any collaborative or strategic partnering efforts, technological advances and the competitive environment for our planned products. As of the date of this prospectus supplement, we cannot specify with certainty all of the particular uses for the net proceeds to us from the sale of the shares of our common stock offered by us hereunder. Accordingly, our management will have broad discretion in the timing and application of these proceeds. Pending application of the net proceeds as described above, we intend to temporarily invest the proceeds in short-term, interest-bearing instruments.
| S-32 |
The following table sets forth our cash and our capitalization as of January 31, 2021:
| · | on an actual basis; and |
| · | on an as adjusted basis to reflect the sale by us in this offering of (i) shares of our common stock and accompanying Common Warrant at the public offering price of $ per share and accompanying Common Warrant and (ii) Pre-funded Warrants and accompanying Common Warrant at the public offering price of $ per Pre-funded Warrant and accompanying Common Warrant, after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. |
You should read the data set forth in the table below in conjunction with our financial statements, including the related notes, and "Management's Discussion and Analysis of Financial Condition and Results of Operations" from our Quarterly Report on Form 10-Q for the quarter ended January 31, 2021, which is incorporated by reference into this prospectus supplement.
| As of January 31, 2021 | ||||||||
Actual | As Adjusted | |||||||
| (unaudited) | ||||||||
| (in thousands, except share data) | ||||||||
| Cash and cash equivalents | $ | 3,091 | $ | |||||
| Total liabilities | $ | 649 | $ | |||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.0001 par value; 10,000,000 shares authorized, no shares issued and outstanding, actual and as adjusted | $ | – | $ | – | ||||
| Common stock, $0.0001 par value; 1,666,667 shares authorized, 1,560,940 shares issued and outstanding, actual; shares issued and outstanding, as adjusted | – | |||||||
| Additional paid-in capital | 114,084 | |||||||
| Accumulated deficit | (106,471 | ) | ||||||
| Total stockholders' equity | $ | 7,613 | $ | |||||
| Total capitalization | $ | 114,092 | $ | |||||
The table and discussion above assumes the exercise of the Pre-funded Warrants and no exercise of the Common Warrants offered and sold in this offering.
The number of shares of our common stock to be outstanding after this offering is based on 1,560,940 shares of our common stock outstanding as of January 31, 2021 (subject to adjustment based on issuances of additional shares as applicable due to the rounding up of fractional shares resulting from the 1:1,500 reverse stock split), and excludes:
| · | 41,734 shares of our common stock issuable upon the exercise of stock options outstanding as of January 31, 2021 at a weighted-average exercise price of $79.96 per share; |
| · | 43,505 shares of our common stock issuable upon the exercise of outstanding warrants as of January 31, 2021 , at a weighted average exercise price of $16.54 per share; |
| · | shares of common stock issuable upon exercise of the Common Warrants issued in this offering; and |
| · | up to shares of common stock (or if the underwriter exercises its option to purchase additional shares of common stock in full, up to shares of common stock) issuable upon exercise of warrants with an exercise price of $ per share to be issued to the underwriter or its designees as compensation in connection with this offering. |
| S-33 |
Purchasers of our common stock, Pre-funded Warrants and Common Warrants in this offering will experience immediate dilution to the extent of the difference between the effective public offering price per share of our common stock, Pre-funded Warrants and/or Common Warrants and the as adjusted net tangible book value per share of our common stock immediately after this offering.
Our net tangible book value as of January 31, 2021 was approximately $2.5 million, or $1.58 per share of our common stock. Net tangible book value per share of our common stock is determined by dividing total tangible assets less total liabilities, excluding items such as intangibles, by the aggregate number of shares of our common stock outstanding as of January 31, 2021.
After giving effect to the sale of (i) shares of our common stock and accompanying Common Warrants to purchase shares of common stock in this offering at a public offering price of $ per share and accompanying Common Warrant and (ii) Pre-funded Warrants to purchase shares of common stock and accompanying Common Warrants to purchase shares of common stock in this offering at a public offering price of $ per Pre-funded Warrant and accompanying Common Warrant, and after deducting underwriting discounts and commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of January 31, 2021 would have been approximately $ , or approximately $ per share of our common stock. This represents an immediate increase in net tangible book value of $ per share of our common stock to our existing stockholders and an immediate dilution in net tangible book value of $ per share of our common stock to purchasers in this offering.
The following table illustrates this calculation on a per share basis:
| Offering price per share and accompanying Common Warrant in this offering | $ | |||||||
| Net tangible book value per share as of January 31, 2021 | $ | 1.58 | ||||||
| Increase in net tangible book value per share attributable to purchasers in this offering | $ | |||||||
| As adjusted net tangible book value per share immediately after this offering | $ | |||||||
| Dilution per share to purchasers in this offering | $ |
If the underwriter exercises its option in full to purchase additional shares of our common stock and/or Common Warrants in this offering at the public offering price of $ per share, the as adjusted net tangible book value per share after the offering would be $ per share, the decrease in the net tangible book value per share to existing stockholders would be $ per share and the dilution to new investors purchasing securities in this offering would be $ per share.
The discussion and table above assume no exercise of Common Warrants and full exercise of the Pre-funded Warrants sold in this offering.
The above table is based on 1,560,940 shares of our common stock outstanding as of January 31, 2021 (subject to adjustment based on issuances of additional shares as applicable due to the rounding up of fractional shares resulting from the 1:1,500 reverse stock split), and excludes:
| · | 41,734 shares of our common stock issuable upon the exercise of stock options outstanding as of January 31, 2021, at a weighted-average exercise price of $79.96 per share; |
| · | 43,505 shares of our common stock issuable upon the exercise of outstanding warrants as of January 31, 2021, at a weighted average exercise price of $16.54 per share; |
| S-34 |
| · | shares of common stock issuable upon exercise of the Common Warrants issued in this offering; and |
| · | up to shares of common stock (or if the underwriter exercises its option to purchase additional shares of common stock in full, up to shares of common stock) issuable upon exercise of warrants with an exercise price of $ per share to be issued to the underwriter or its designees as compensation in connection with this offering. |
To the extent that options or warrants are exercised, other equity awards vest, new equity awards are issued under the 2021 Equity Incentive Plan or pursuant to inducement awards, or we issue additional shares of common stock in the future, there may be further dilution to investors participating in this offering. In addition, we may choose to raise additional capital because of market conditions or strategic considerations, even if we believe that we have sufficient funds for our current or future operating plans. If we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
| S-35 |
MATERIAL U.S. FEDERAL INCOME TAX CONSIDERATIONS FOR NON-U.S. HOLDERS
The following discussion is a summary of certain material U.S. federal income tax considerations for non-U.S. holders (as defined below) with respect to their ownership and disposition of shares of our common stock issued pursuant to this offering. For purposes of this discussion, a “non-U.S. holder is a beneficial owner of our common stock that, for U.S. federal income tax purposes is neither a “U.S. person” nor an entity (or arrangement) treated as a partnership. A “U.S. person” is any person that, for U.S. federal income tax purposes, is or is treated as any of the following:
| · | an individual who is a citizen or resident of the United States; |
| · | a corporation or other entity taxable as a corporation created or organized in the United States or under the laws of the United States or any political subdivision thereof, or otherwise treated as such for U.S. federal income tax purposes; |
| · | an estate whose income is subject to U.S. federal income tax regardless of its source; or |
| · | a trust (x) whose administration is subject to the primary supervision of a U.S. court and that has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (y) that has made a valid election under applicable Treasury Regulations to be treated as a U.S. person. |
This discussion does not address the tax treatment of partnerships or other entities that are pass-through entities for U.S. federal income tax purposes or persons that hold their common stock through partnerships or other pass-through entities. A partner in a partnership or other pass-through entity that will hold our common stock should consult his, her or its tax advisor regarding the tax consequences of acquiring, holding and disposing of our common stock through a partnership or other pass-through entity, as applicable.
This discussion is based on current provisions of the U.S. Internal Revenue Code of 1986, as amended, which we refer to as the Code, existing and proposed U.S. Treasury Regulations promulgated thereunder, current administrative rulings and judicial decisions, all as in effect as of the date of this prospectus and all of which are subject to change or to differing interpretation, possibly with retroactive effect. Any such change or differing interpretation could alter the tax consequences to non-U.S. holders described in this prospectus supplement. There can be no assurance that the Internal Revenue Service, which we refer to as the IRS, will not challenge one or more of the tax consequences described herein. We assume in this discussion that a non-U.S. holder holds shares of our common stock as a capital asset within the meaning of Section 1221 of the Code, generally property held for investment.
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to a particular non-U.S. holder in light of that non-U.S. holder’s individual circumstances nor does it address U.S. state, local or non-U.S. taxes, the alternative minimum tax, the rules regarding qualified small business stock within the meaning of Section 1202 of the Code, the Medicare tax on net investment income or any other aspect of any U.S. federal tax other than the income tax. This discussion also does not consider any specific facts or circumstances that may apply to a non-U.S. holder and does not address the special tax rules applicable to particular non-U.S. holders, such as:
| · | insurance companies; |
| · | tax-exempt or governmental organizations; |
| · | financial institutions; |
| · | brokers or dealers in securities; |
| · | regulated investment companies; |
| S-36 |
| · | pension plans; |
| · | “controlled foreign corporations,” “passive foreign investment companies,” and corporations that accumulate earnings to avoid U.S. federal income tax; |
| · | “qualified foreign pension funds” as defined in Section 897(I)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds; |
| · | persons deemed to sell our common stock under the constructive sale provisions of the Code; |
| · | persons that hold our common stock as part of a straddle, hedge, conversion transaction, synthetic security or other integrated investment; |
| · | persons who hold or receive our common stock pursuant to the exercise of any employee stock option or otherwise as compensation; and |
| · | certain U.S. expatriates. |
This discussion is for general information only and is not tax advice. Accordingly, all prospective non-U.S. holders of our common stock should consult their tax advisors with respect to the U.S. federal, state, local and non-U.S. tax consequences of the ownership and disposition of our common stock.
Distributions on Our Common Stock
As described in the “Risk Factors” section above, we do not anticipate paying any cash dividends on our capital stock in the foreseeable future. Distributions, if any, on our common stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. If a distribution exceeds our current and accumulated earnings and profits, the excess will be treated as a tax-free return of the non-U.S. holder’s investment, up to such holder’s tax basis in the common stock. Any remaining excess will be treated as capital gain, subject to the tax treatment described below in “Gain on Sale or Other Taxable Disposition of Our Common Stock.” Any such distributions will also be subject to the discussions below under the sections titled “Backup Withholding and Information Reporting” and “FATCA.”
Subject to the discussion in the following two paragraphs in this section, dividends paid to a non-U.S. holder generally will be subject to withholding of U.S. federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty between the U.S. and such holder’s country of residence.
Dividends that are treated as effectively connected with a trade or business conducted by a non-U.S. holder within the U.S. are generally exempt from the 30% withholding tax if the non-U.S. holder satisfies applicable certification and disclosure requirements. However, such U.S. effectively connected income, net of specified deductions and credits, is taxed at the same graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code), subject to an applicable income tax treaty providing otherwise. Any U.S. effectively connected income received by a non-U.S. holder that is a corporation may also, under certain circumstances, be subject to an additional “branch profits tax” at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty between the U.S. and such holder’s country of residence, on its effectively connected earnings and profits that are not reinvested in the United States.
A non-U.S. holder of our common stock who claims the benefit of an applicable income tax treaty between the U.S. and such holder’s country of residence generally will be required to provide a properly executed IRS Form W-8BEN or W-8BEN-E (or successor form) to the applicable withholding agent and satisfy applicable certification and other requirements. Non-U.S. holders are urged to consult their tax advisors regarding their entitlement to benefits under a relevant income tax treaty. A non-U.S. holder that is eligible for a reduced rate of U.S. withholding tax under an income tax treaty may obtain a refund or credit of any excess amounts withheld by timely filing a U.S. tax return with the IRS.
| S-37 |
Gain on Sale or Other Taxable Disposition of Our Common Stock
Subject to the discussions below under “Backup Withholding and Information Reporting” and “FATCA,” a non-U.S. holder generally will not be subject to any U.S. federal income or withholding tax on any gain realized upon such holder’s sale or other taxable disposition of shares of our common stock unless:
| · | the gain is effectively connected with the non-U.S. holder’s conduct of a U.S. trade or business and, if an applicable income tax treaty so provides, is attributable to a permanent establishment or a fixed base maintained by such non-U.S. holder in the U.S., in which case the non-U.S. holder generally will be taxed on a net income basis at the graduated U.S. federal income tax rates applicable to U.S. persons (as defined in the Code) and, if the non-U.S. holder is a foreign corporation, the branch profits tax described above in “Distributions on Our Common Stock” also may apply; |
| · | the non-U.S. holder is a nonresident alien individual who is present in the U.S. for 183 days or more in the taxable year of the disposition and certain other conditions are met, in which case the non-U.S. holder will be subject to a 30% tax (or such lower rate as may be specified by an applicable income tax treaty between the U.S. and such holder’s country of residence) on the net gain derived from the disposition, which may be offset by certain U.S. source capital losses of the non-U.S. holder; or |
| · | we are, or have been, at any time during the five-year period preceding such sale or other taxable disposition (or the non-U.S. holder’s holding period, if shorter) a U.S. real property holding corporation, unless our common stock is regularly traded on an established securities market and the non-U.S. holder holds no more than 5% of our outstanding common stock, directly or indirectly, actually or constructively, during the shorter of the 5-year period ending on the date of the disposition or the period that the non-U.S. holder held our common stock. Generally, a corporation is a U.S. real property holding corporation only if the fair market value of its U.S. real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide real property interests plus its other assets used or held for use in a trade or business. Although there can be no assurance, we do not believe that we are, or have been, a U.S. real property holding corporation, or that we are likely to become one in the future. No assurance can be provided that our common stock will be regularly traded on an established securities market for purposes of the rules described above. |
Backup Withholding and Information Reporting
We must report annually to the IRS and to each non-U.S. holder the gross amount of the distributions on our common stock paid to such holder and the tax withheld, if any, with respect to such distributions. Non-U.S. holders may have to comply with specific certification procedures to establish that the holder is not a United States person (as defined in the Code) in order to avoid backup withholding at the applicable rate with respect to dividends on our common stock. Dividends paid to non-U.S. holders subject to withholding of U.S. federal income tax, as described above in “Distributions on Our Common Stock,” generally will be exempt from U.S. backup withholding.
Information reporting and backup withholding will generally apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the U.S. through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker.
Non-U.S. holders should consult their tax advisors regarding the application of the information reporting and backup withholding rules to them. Copies of information returns may be made available to the tax authorities of the country in which the non-U.S. holder resides or is incorporated under the provisions of a specific treaty or agreement. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a non-U.S. holder can be refunded or credited against the non-U.S. holder’s U.S. federal income tax liability, if any, provided that an appropriate claim is filed with the IRS in a timely manner.
| S-38 |
FATCA
Provisions of the Code commonly referred to as the Foreign Account Tax Compliance Act, or FATCA, generally impose a U.S. federal withholding tax at a rate of 30% on payments of dividends on our common stock paid to a foreign entity unless (i) if the foreign entity is a “foreign financial institution,” such foreign entity undertakes certain due diligence, reporting, withholding, and certification obligations, (ii) if the foreign entity is not a “foreign financial institution,” such foreign entity identifies certain of its U.S. investors, if any, or (iii) the foreign entity is otherwise exempt under FATCA. Such withholding may also apply to gross proceeds from the sale or other disposition of our common stock, although under recently proposed U.S. Treasury Regulations, no withholding would apply to such gross proceeds. The preamble to the proposed regulations specifies that taxpayers (including withholding agents) are permitted to rely on the proposed regulations pending finalization. Under certain circumstances, a non-U.S. holder may be eligible for refunds or credits of this withholding tax. An intergovernmental agreement between the U.A. and an applicable foreign country may modify the requirements described in this paragraph. Non-U.S. holders should consult their tax advisors regarding the possible implications of this legislation on their investment in our common stock and the entities through which they hold our common stock, including, without limitation, the process and deadlines for meeting the applicable requirements to prevent the imposition of the 30% withholding tax under FATCA.
The preceding discussion of U.S. federal income tax considerations is for general information only. It is not tax advice. Each prospective investor should consult its own tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of purchasing, holding and disposing of our common stock, including the consequences of any proposed change in applicable laws.
| S-39 |
We have entered into an underwriting agreement with H.C. Wainwright & Co., LLC, as underwriter, with respect to the shares of common stock, Pre-funded Warrants and accompanying Common Warrants being offered in this offering. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase from us, at the respective public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, shares of our common stock, Pre-funded Warrants to purchase shares of common stock and accompanying Common Warrants to purchase shares of common stock.
| Underwriters | Number of Shares of Common Stock | Number of Pre-funded Warrants | Number of Common Warrants | |||||||||
| H.C. Wainwright & Co., LLC | ||||||||||||
| Total |
Pursuant to the underwriting agreement, the underwriter has agreed to purchase all of the shares, Pre-Funded Warrants and accompanying Common Warrants sold under the underwriting agreement if any of these shares, Pre-funded Warrants and accompanying Common Warrants are purchased, other than those shares covered by the underwriter’s option to purchase additional shares of common stock and/or Common Warrants described below. The underwriter has advised us that it does not intend to confirm sales to any account over which it exercises discretionary authority. We have agreed to indemnify the underwriter against certain liabilities, including liabilities under the Securities Act of 1933, as amended (“Securities Act”), or to contribute to payments the underwriter may be required to make in respect of those liabilities.
The underwriter is offering the shares of common stock, the Pre-Funded Warrants and the accompanying Common Warrants subject to its acceptance of the shares of common stock, Pre-funded Warrants and accompanying Common Warrants from us and subject to prior sale. The underwriting agreement provides that the obligations of the underwriter to pay for and accept delivery of the securities offered by this prospectus supplement are subject to the approval of certain legal matters by their counsel and to certain other conditions specified in the underwriting agreement. If all of the shares of common stock, the Pre-funded Warrants and the accompanying Common Warrants are not sold at the public offering prices, the underwriter may change the offering price and other selling terms.
Commissions and Discounts; Expenses
The underwriter has advised us that they propose initially to offer the shares of common stock, the Pre-funded Warrants and the accompanying Common Warrants to the public at the public offering price set forth on the cover of this prospectus supplement and to dealers at that price less a concession not in excess of $ per share and accompanying Common Warrant. After the initial offering, the public offering price, concession or any other term of the offering may be changed.
The following table shows the public offering price per share of common stock and accompanying Common Warrant and price per Pre-funded Warrant and accompanying Common Warrant and total public offering price, underwriting discounts and commissions, and proceeds before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriter’s option to purchase up to additional shares of common stock and Common Warrants to purchase up to shares of common stock (but without giving effect to any exercise of such additional Common Warrants issuable upon exercise of the underwriter’s option).
| Total | ||||||||||||||||
| Per Share and accompanying Common Warrant | Per Pre-funded Warrant and Accompanying Common Warrant | No Exercise | Full Exercise | |||||||||||||
| Public offering price | ||||||||||||||||
| Underwriting discounts and commissions to be paid by us | ||||||||||||||||
| Proceeds, before expenses, to us | ||||||||||||||||
| S-40 |
In addition, we have agreed to issue to the underwriter or its designees warrants to purchase up to shares of common stock (or if the underwriter exercises its option to purchase additional shares of common stock in full, up to shares of common stock) (representing 7.5% of the aggregate number of shares of common stock and Pre-funded Warrants sold in this offering), at an exercise price of $ per share (representing 125% of the public offering price for a share of common stock and the accompanying warrant to be sold in this offering). The underwriter warrants will be exercisable immediately and for years from the date of issuance of the underwriter warrants. The issuance of the underwriter warrants and the shares issuable upon exercise of the underwriter warrants are registered on the registration statement of which this prospectus forms a part.
We estimate expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $ , which includes certain expenses incurred by the underwriter in connection with this offering that will be reimbursed by us. We have agreed to reimburse the underwriter for certain expenses incurred by the underwriter in connection with this offering including certain fees and expenses of counsel for the underwriter in an amount not to exceed $100,000, $50,000 for nonaccountable expenses and clearing fees of $15,950. In addition, we have also agreed to pay the underwriter a management fee equal to 1% of the aggregate gross proceeds in this offering.
Option to Purchase Additional Shares and/or Common Warrants
We have granted the underwriter an option to purchase up to additional shares of common stock and/or Common Warrants (up to 15% of the shares of common stock and shares underlying the Pre-funded Warrants sold in this offering) at the public offering price, less the underwriting discounts and commissions, within 30 days from the date of this prospectus supplement.
Right of First Refusal
We have granted the underwriter a 18-month right of first refusal to act as exclusive financial advisor, sole book-running manager, sole underwriter, or sole placement agent, as applicable, if we or any of our subsidiaries decides to dispose or acquire businesses, finance or refinance any indebtedness or raise funds by means of a public offering or a private placement or any other capital-raising financing of equity, equity-linked or debt securities using an underwriter or placement agent other than with respect to certain excluded transactions. If the underwriter or an affiliate of the underwriter accepts any such engagement, the agreement governing such engagement will contain, among other things, provisions for customary fees and terms for transactions of similar size and nature, including indemnification, which are appropriate to such a transaction.
Tail
In the event that any investors that were contacted or were introduced to us by the underwriter during the term of its engagement provide any capital to us in a public or private offering or capital-raising transaction during the twelve-month period following the expiration or termination of the engagement of the underwriter, we shall pay the underwriter the cash and warrant compensation provided above on the gross proceeds from such investors.
No Sales of Similar Securities; Lock-ups
We have agreed to not sell any shares of our common stock or any securities convertible into or exercisable or exchangeable into shares of common stock, subject to certain exceptions, for a period of 90 days after the pricing of this offering unless we obtain prior written consent of the underwriter. This consent may be given at any time without public notice, and the underwriter may consent in its sole discretion. The exceptions to the restriction include, among other things, the issuance of any shares of our capital stock or securities convertible into shares of our capital stock that are issued (i) pursuant to a stock or option plan, (ii) pursuant to the exercise or exchange of or conversion of any securities exercisable or exchangeable for or convertible into shares of common stock issued and outstanding on the date of this prospectus supplement, and (iii) as consideration in an acquisition, merger or similar strategic transaction approved by a majority of the disinterested directors, provided that such securities are issued as “restricted securities” as defined in Rule 144 and carry no registration rights that require or permit the filing of any registration statement in connection therewith within 90 days following the pricing of this offering, and provided that any such issuance shall only be to a person providing us business synergies and additional benefits in addition to the investment of funds, but shall not include a transaction in which we are issuing securities primarily for the purpose of raising capital or to an entity whose primary business is investing in securities.
| S-41 |
We have also agreed, subject to certain exceptions, until the one-year anniversary of the first closing date of this offering, not to (i) issue or sell any debt or equity securities that are convertible into, exchangeable or exercisable for, or include the right to receive, additional shares of common stock either (A) at a conversion price, exercise price or exchange rate or other price that is based upon, and/or varies with, the trading prices of or quotations for the shares of common stock at any time after the initial issuance of such debt or equity securities or (B) with a conversion, exercise or exchange price that is subject to being reset at some future date after the initial issuance of such debt or equity security or upon the occurrence of specified or contingent events directly or indirectly related to our business or the market for our common stock or (ii) enter into, or effect a transaction under, any agreement, including, but not limited to, an equity line of credit, whereby we may issue securities at a future determined price.
In addition, each of our directors and executive officers has entered into a lock-up agreement with the underwriter. Under the lock-up agreements, subject to certain limited circumstances, the directors and executive officers may not, directly or indirectly, sell, offer to sell, contract to sell, or grant any option for the sale (including any short sale), grant any security interest in, pledge, hypothecate, hedge, or otherwise dispose of, or enter into any transaction which is designed to or could be expected to result in the disposition of, any shares of our common stock or securities convertible into or exchangeable for shares of our common stock, or publicly announce any intention to do any of the foregoing, unless such directors and executive officers obtain prior written consent of the underwriter for a period of 90 days from the date of this prospectus supplement. This consent may be given at any time without public notice, and the underwriter may consent in its sole discretion. Such lock-up restriction applies to all shares owned by our directors and executive officers whether owned as of the date of this prospectus supplement or subsequently acquired.
Nasdaq Capital Market Listing
Our common stock has been approved for listing on Nasdaq under the symbol “PMCB.” There is no established trading market for the Pre-funded Warrants or the Common Warrants, and we do not expect a trading market to develop. We do not intend to list the Pre-funded Warrants or the Common Warrants on any securities exchange or nationally recognized trading system. Without a trading market, the liquidity of the Pre-funded Warrants and the Common Warrants will be extremely limited.
Price Stabilization, Short Positions and Penalty Bids
In connection with this offering, the underwriter may engage in stabilizing transactions, overallotment transactions, syndicate covering transactions and penalty bids in connection with our common stock.
Stabilizing transactions permit bids to purchase shares of common stock so long as the stabilizing bids do not exceed a specified maximum.
Overallotment transactions involve sales by the underwriter of shares of common stock in excess of the number of shares the underwriter is obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriter is not greater than the number of shares that it may purchase in the overallotment option. In a naked short position, the number of shares involved is greater than the number of shares in the overallotment option. The underwriter may close out any short position by exercising its overallotment option and/or purchasing shares in the open market.
Syndicate covering transactions involve purchases of common stock in the open market after the distribution has been completed in order to cover syndicate short positions. Such a naked short position would be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering.
Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions.
| S-42 |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock or preventing or retarding a decline in the market price of our common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriter make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected on Nasdaq, in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
In connection with this offering, the underwriter also may engage in passive market making transactions in our common stock in accordance with Regulation M during the period before the commencement of offers or sales of shares of our common stock in this offering and extending through the completion of the distribution. In general, a passive market maker must display its bid at a price not in excess of the highest independent bid for that security. However, if all independent bids are lowered below the passive market maker’s bid that bid must then be lowered when specific purchase limits are exceeded. Passive market making may stabilize the market price of the securities at a level above that which might otherwise prevail in the open market and, if commenced, may be discontinued at any time.
Electronic Distribution
A prospectus in electronic format may be made available on the websites, if any, maintained by the underwriter participating in this offering and the underwriter may distribute prospectuses electronically. Other than the prospectus in electronic format, the information on these websites is not part of this prospectus or the registration statement of which this prospectus form a part, has not been approved or endorsed by us or the underwriter, and should not be relied upon by investors.
Other Relationships
From time to time, certain of the underwriter and its affiliates have provided, and may provide in the future, various advisory, investment and commercial banking and other services to us in the ordinary course of business, for which they have received and may continue to receive customary fees and commissions.
Transfer Agent
The transfer agent and registrar for our common stock is American Stock Transfer & Trust Company, LLC.
Listing
The Company’s OTCQB Market Symbol is PMCB, although as a result of the recent reverse split of the Company’s common stock, until August 6, 2021, the Company’s OTCQB Market Symbol has temporarily been “PMCBD.”
Our common stock has been approved for listing on Nasdaq under the symbol “PMCB.” Our common stock will continue to trade on the OTCQB Venture Market until trading commences on Nasdaq, which we expect to occur following the pricing of this offering. The offering, however, is subject to market and other conditions, and there can be no assurance as to whether or when the offering may be completed.
Selling Restrictions
European Economic Area
In relation to each Member State of the European Economic Area (each a “Relevant State”), no securities have been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the securities which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that the securities may be offered to the public in that Relevant State at any time:
| S-43 |
(a) to any legal entity which is a qualified investor as defined under Article 2 of the Prospectus Regulation;
(b) to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or
(c) in any other circumstances falling within Article 1(4) of the Prospectus Regulation,
provided that no such offer of the securities shall require us or any underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to the securities in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase or subscribe for any securities, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
United Kingdom
No securities have been offered or will be offered pursuant to the offering to the public in the United Kingdom prior to the publication of a prospectus in relation to the securities which has been approved by the Financial Conduct Authority, except that the securities may be offered to the public in the United Kingdom at any time:
(a) to any legal entity which is a qualified investor as defined under Article 2 of the UK Prospectus Regulation;
(b) to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the UK Prospectus Regulation), subject to obtaining the prior consent of the representatives for any such offer; or
(c) in any other circumstances falling within Section 86 of the Financial Services and Markets Act 2000 (“FSMA”).
provided that no such offer of the securities shall require us or any underwriter to publish a prospectus pursuant to Section 85 of the FSMA or supplement a prospectus pursuant to Article 23 of the UK Prospectus Regulation. For the purposes of this provision, the expression an “offer to the public” in relation to the securities in the United Kingdom means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase or subscribe for any securities and the expression “UK Prospectus Regulation” means Regulation (EU) 2017/1129 as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018.
Canada
The securities may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (“NI 33-105”), the underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering. Pursuant to section 3A.3 (or, in the case of securities issued or guaranteed by the government of a non-Canadian jurisdiction, section 3A.4) of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
| S-44 |
Switzerland
This document is not intended to constitute an offer or solicitation to purchase or invest in the securities described herein. The securities may not be publicly offered, sold or advertised, directly or indirectly, in, into or from Switzerland and will not be listed on the SIX Swiss Exchange or on any other exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the securities constitutes a prospectus as such term is understood pursuant to article 652a or article 1156 of the Swiss Code of Obligations or a listing prospectus within the meaning of the listing rules of the SIX Swiss Exchange or any other regulated trading facility in Switzerland, and neither this document nor any other offering or marketing material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, nor us nor the securities have been or will be filed with or approved by any Swiss regulatory authority. The securities are not subject to the supervision by any Swiss regulatory authority, e.g., the Swiss Financial Markets Supervisory Authority, and investors in the securities will not benefit from protection or supervision by such authority.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by the Israel Securities Authority. In Israel, this prospectus is being distributed only to, and is directed only at, and any offer of the ordinary securities is directed only at (i) a limited number of persons in accordance with the Israeli Securities Law and (ii) investors listed in the first addendum (“the Addendum”) to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriter, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals,” each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case, purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors are required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
| S-45 |
DESCRIPTION OF SECURITIES WE ARE OFFERING
Common Stock
We are offering shares of our common stock in this offering. As of July 31, 2021, there were 1,591,420 shares of common stock issued and outstanding, held of record by approximately 35,000 stockholders. See “Description of Securities we may Offer” in our prospectus for more information regarding our shares of common stock.
Pre-funded Warrants
The following summary of certain terms and provisions of the Pre-funded Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Pre-funded Warrant, the form of which will be filed as an exhibit to a Current Report on Form 8-K in connection with this offering and incorporated by reference into the registration statement of which this prospectus supplement forms a part. Prospective investors should carefully review the terms and provisions of the form of Pre-funded Warrant for a complete description of the terms and conditions of the Pre-funded Warrants.
Pre-funded warrants will be issued in certificated form only.
Duration and Exercise Price
Each Pre-funded Warrant offered hereby will have an initial exercise price per share equal to $0.001. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
The Pre-funded Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s Pre-funded Warrant to the extent that the holder would own more than 4.99% (or at the election of the holder, 9.99%) of the outstanding shares of common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding shares of common stock after exercising the holder’s Pre-funded Warrants up to 9.99% of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Pre-funded Warrants. No fractional shares of common stock will be issued in connection with the exercise of a Pre-funded Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless Exercise
In lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Pre-funded Warrants.
Fundamental Transactions
In the event of any fundamental transaction, as described in the Pre-funded Warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our shares of common stock, then upon any subsequent exercise of a Pre-funded Warrant, the holder will have the right to receive as alternative consideration, for each share of common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation or of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of common stock for which the Pre-funded Warrant is exercisable immediately prior to such event.
| S-46 |
Transferability
Subject to applicable laws, a Pre-funded Warrant may be transferred at the option of the holder upon surrender of the Pre-funded Warrant to us together with the appropriate instruments of transfer and payment of funds sufficient to pay any transfer taxes (if applicable).
Exchange Listing
There is no established trading market for the Pre-funded Warrants. We do not intend to list the Pre-funded Warrants on any securities exchange or nationally recognized trading system.
Right as a Shareholder
Except as otherwise provided in the Pre-funded Warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the Pre-funded Warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until such Pre-funded Warrant holders exercise their Pre-funded Warrants.
Common Warrants
The following summary of certain terms and provisions of the Common Warrants that are being offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the Common Warrant, the form of which will be filed as an exhibit to a Current Report on Form 8-K in connection with this offering and incorporated by reference into the registration statement of which this prospectus supplement forms a part. Prospective investors should carefully review the terms and provisions of the form of Common Warrant for a complete description of the terms and conditions of the Common Warrants.
Common Warrants will be issued in certificated form only.
Duration and Exercise Price
Each Common Warrant offered hereby has an initial exercise price per share equal to $ . The Common Warrants are immediately exercisable and will expire on the anniversary of the original issuance date. The exercise price and number of shares of common stock issuable upon exercise is subject to appropriate adjustment in the event of stock dividends, stock splits, reorganizations or similar events affecting our common stock and the exercise price.
Exercisability
The Common Warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise as discussed below). A holder (together with its affiliates) may not exercise any portion of such holder’s Common Warrant to the extent that the holder would own more than 4.99% (or, at the election of the holder, 9.99%) of the outstanding shares of common stock immediately after exercise, except that upon at least 61 days’ prior notice from the holder to us, the holder may increase the amount of ownership of outstanding shares of common stock after exercising the holder’s Common Warrants up to 9.99% of the number of shares of common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the Common Warrants. No fractional shares of common stock will be issued in connection with the exercise of a Common Warrant. In lieu of fractional shares, we will either pay the holder an amount in cash equal to the fractional amount multiplied by the exercise price or round up to the next whole share.
Cashless Exercise
If, at the time a holder exercises its Common Warrants, a registration statement registering the issuance of the shares of common stock underlying the Common Warrants under the Securities Act is not then effective or available, then in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, the holder may elect instead to receive upon such exercise (either in whole or in part) the net number of shares of common stock determined according to a formula set forth in the Common Warrants.
| S-47 |
Fundamental Transaction
In the event of any fundamental transaction, as described in the Common Warrants and generally including any merger with or into another entity, sale of all or substantially all of our assets, tender offer or exchange offer, or reclassification of our shares of common stock, then upon any subsequent exercise of a Common Warrant, the holder will have the right to receive as alternative consideration, for each share of common stock that would have been issuable upon such exercise immediately prior to the occurrence of such fundamental transaction, the number of shares of common stock of the successor or acquiring corporation of our company, if it is the surviving corporation, and any additional consideration receivable upon or as a result of such transaction by a holder of the number of shares of common stock for which the Common Warrant is exercisable immediately prior to such event. Notwithstanding the foregoing, in the event of a fundamental transaction, the holders of the Common Warrants have the right to require us or a successor entity to redeem the Common Warrants for cash in the amount of the Black Scholes Value (as defined in each Common Warrant) of the unexercised portion of the Common Warrants concurrently with or within 30 days following the consummation of a fundamental transaction. However, in the event of a fundamental transaction which is not in our control, including a fundamental transaction not approved by our board of directors, the holders of the Common Warrants will only be entitled to receive from us or our successor entity, as of the date of consummation of such fundamental transaction the same type or form of consideration (and in the same proportion), at the Black Scholes Value of the unexercised portion of the Common Warrant, that is being offered and paid to the holders of our common stock in connection with the fundamental transaction, whether that consideration is in the form of cash, stock or any combination of cash and stock, or whether the holders of our common stock are given the choice to receive alternative forms of consideration in connection with the fundamental transaction.
Transferability
Subject to applicable laws, a Common Warrant may be transferred at the option of the holder upon surrender of the Common Warrant to us together with the appropriate instruments of transfer and payment of funds sufficient to pay any transfer taxes (if applicable).
Exchange Listing
There is no trading market available for the Common Warrants on any securities exchange or nationally recognized trading system. We do not intend to list the Common Warrants on any securities exchange or nationally recognized trading system.
Right as a Shareholder
Except as otherwise provided in the Common Warrants or by virtue of such holder’s ownership of shares of our common stock, the holders of the Common Warrants do not have the rights or privileges of holders of our common stock, including any voting rights, until they exercise their Common Warrants.
Underwriter Warrants
We are also registering hereunder the Underwriter Warrants and the shares of common stock issuable upon exercise thereof which we have agreed to grant to H.C. Wainwright & Co., LLC, or its designees, as partial consideration for the underwriting services. The Underwriter Warrants are exercisable for a number of our shares equal to 7.5% of the aggregate number of shares and Pre-funded Warrants sold to the investors in this offering. The Underwriter Warrants will have the same terms as the Common Warrants described above, except that the Underwriter Warrants will have an exercise price of $ (125% of the public offering price per share and the accompanying warrant) and will terminate years after the date of commencement of sales in this offering.
Reference is hereby made to the “—Common Warrants” description above and to the provisions of the Underwriter Warrant, the form of which will be filed with the SEC as an exhibit to a Current Report on Form 8-K in connection with this offering and incorporated by reference into the registration statement of which this prospectus supplement and the accompanying prospectus form a part.
| S-48 |
Certain legal matters in connection with this offering will be passed upon for us by Troutman Pepper Hamilton Sanders LLP, New York, New York. Certain legal matters governed by Nevada law with respect to the validity of the offered securities will be passed upon for us by Ballard Spahr LLP, Las Vegas, Nevada. Haynes and Boone, LLP, New York, New York, is acting as counsel for the underwriter in connection with certain legal matters related to this offering.
| S-49 |
The consolidated financial statements of PharmaCyte Biotech, Inc. as of April 30, 2020 and 2019, and for each of the years in the two-year period ended April 30, 2020, have been incorporated by reference herein in reliance upon the report of Armanino LLP, the Company’s independent registered public accounting firm, incorporated by reference herein and upon the authority of Armanino LLP as experts in accounting and auditing.
| S-50 |
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act, of which this prospectus supplement forms a part. The rules and regulations of the SEC allow us to omit from this prospectus supplement certain information included in the registration statement. For further information about us and the securities we are offering under this prospectus supplement, you should refer to the registration statement and the exhibits and schedules filed with the registration statement. With respect to the statements contained in this prospectus supplement regarding the contents of any agreement or any other document, in each instance, the statement is qualified in all respects by the complete text of the agreement or document, a copy of which has been filed as an exhibit to the registration statement.
Because we are subject to the information and reporting requirements of the Exchange Act, we file annual, quarterly and current reports and other information with the SEC. Our SEC filings are available to the public over the Internet at the SEC’s website at http://www.sec.gov.
We make available free of charge on our website our annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after we electronically file such material with, or furnish such material to, the SEC. Please note, however, that we have not incorporated any other information by reference from our website, other than the documents listed under the heading “Incorporation of Certain Information by Reference” on page S-52 of this prospectus supplement. In addition, you may request copies of these filings at no cost by writing or telephoning us at the following address or telephone number:
PharmaCyte Biotech, Inc.,
23046 Avenida de la Carlota, Suite 600,
Laguna Hills, California 92653
(917) 595-2850
| S-51 |
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
SEC rules permit us to incorporate information by reference in this prospectus supplement. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be part of this prospectus supplement, except for information superseded by information contained in this prospectus supplement itself or in any subsequently filed incorporated document. This prospectus supplement incorporates by reference the documents set forth below that we have previously filed with the SEC, other than information in such documents that is deemed to be furnished and not filed. These documents contain important information about us and our business and financial condition.
| · | our Annual Report on Form 10-K for the fiscal year ended April 30, 2020, filed with the SEC on August 13, 2020; |
| · | our Quarterly Reports on Form 10-Q for the fiscal quarters ended July 31, 2020, October 31, 2020 and January 31, 2021, filed with the SEC on September 11, 2020, December 11, 2020 and March 12, 2021, respectively; |
| · | our Current Reports on Form 8-K filed with the SEC on October 16, 2020, May 6, 2021, June 4, 2021, July 6, 2021, and July 13, 2021; and |
| · | The description of our common stock set for in the registration statement on Form 8-A registering our common stock under Section 12 of the Exchange Act, which was filed with the SEC on July 30, 2021, including any amendments or reports filed for purposes of updating such description. |
All documents that we file (but not those that we furnish) pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act, after the date of the initial registration statement of which this prospectus supplement is a part shall be deemed to be incorporated by reference into this prospectus and will automatically update and supersede the information in this prospectus supplement, and any previously filed documents. All documents that we file (but not those that we furnish) pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act on or after the date of this prospectus supplement and prior to the termination of the offering of any of the securities covered under the prospectus shall be deemed to be incorporated by reference into this prospectus supplement and will automatically update and supersede the information in this prospectus supplement and any previously filed documents.
Any statement contained herein or in a document incorporated or deemed to be incorporated by reference in this prospectus supplement shall be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained in the prospectus or this prospectus supplement, or in any other subsequently filed document which also is or is deemed to be incorporated by reference in the prospectus and this prospectus supplement, modifies or supersedes such earlier statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of the prospectus or this prospectus supplement.
To obtain copies of these filings, see “Where You Can Find More Information” on page S-51 of this prospectus supplement.
| S-52 |
PROSPECTUS

$100,000,000.00
PHARMACYTE BIOTECH, INC.
Common Stock
Preferred Stock
Debt Securities
Warrants
Rights
Units
We may offer and sell, from time to time in one or more offerings, up to $100,000,000.00 of our common stock, preferred stock, debt securities, warrants and rights, or any combination of these securities, and/or units consisting of one or more of these securities. We may also offer common stock or preferred stock upon conversion of debt securities or exercise of warrants and common stock upon conversion of preferred stock. All of the securities listed above may be sold separately or as units with other securities.
This prospectus describes some of the general terms that may apply to these securities. When we decide to sell a particular class or series of securities, we will provide specific terms of the offered securities in one or more prospectus supplements. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings.
The prospectus supplement, and any documents incorporated by reference, may also add, update or change information contained in or incorporated by reference into this prospectus. However, no prospectus supplement shall offer a security that is not registered and described in this prospectus at the time of its effectiveness. You should read carefully this prospectus and any prospectus supplement, as well as the documents incorporated by reference or deemed to be incorporated by reference into this prospectus, and any free writing prospectus carefully before you invest. This prospectus may not be used to offer or sell our securities unless accompanied by a prospectus supplement relating to the offered securities.
Our common stock is quoted on the OTCQB under the symbol “PMCB.” Each prospectus supplement will contain information, where applicable, as to our listing on any securities exchange of the securities covered by the prospectus supplement. The aggregate market value of our outstanding common stock held by non-affiliates was $76,316,030 based on 2,341,410,405 shares of outstanding common stock, of which 70,100,000 shares are held by affiliates, and a price of $.03360 per share, which was the last reported sale price of our common stock as quoted on the OTCQB on February 22, 2021.
These securities may be sold by us directly to purchasers, through dealers or agents, or to or through underwriters, or through a combination of these methods. See “Plan of Distribution” in this prospectus. We may also describe the plan of distribution for any particular offering of our securities in a prospectus supplement. If any agents, underwriters or dealers are involved in the sale of any securities in respect of which this prospectus is being delivered, we will disclose their names and the nature of our arrangements with them in a prospectus supplement. The net proceeds we expect to receive from any such sale will also be included in a prospectus supplement.
An investment in our securities involves a high degree of risk. See the sections entitled “Risk Factors” in our most recent Annual Report on Form 10-K, in any Quarterly Report on Form 10-Q and in any Periodic Report on Form 8-K, as well as in any prospectus supplement or free writing prospectus related to these specific offerings.
We may amend or supplement this prospectus from time to time
by filing amendments or supplements as required or related free writing prospectuses. You should read the entire prospectus and
any amendments or supplements carefully before you make your investment decision.
Neither the Securities and Exchange Commission ( “SEC”) nor any state securities commission has approved or disapproved
of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal
offense.
The date of this prospectus is April 14, 2021
| i |
This prospectus is part of a Registration Statement that we filed with the Securities and Exchange Commission using a “shelf” registration process. Under this shelf registration process, we may offer from time to time securities described in this prospectus having a maximum aggregate offering price of $100,000,000.00 in one or more offerings. Each time we offer securities, we will prepare and file with the SEC a prospectus supplement or information that is incorporated by reference into this prospectus that describes the specific amounts, prices and terms of the securities we offer. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings and securities. The prospectus supplement also may add, update or change information contained in this prospectus or the documents incorporated herein by reference. You should read carefully this prospectus, any applicable prospectus supplement and any related free writing prospectus together with additional information described below under the caption “Where You Can Find More Information.”
This prospectus does not contain all the information provided in the Registration Statement we filed with the SEC. For further information about us or our securities offered hereby, you should refer to that Registration Statement, which you can obtain from the SEC as described below under “Where You Can Find More Information.”
You should rely only on the information contained or incorporated by reference in this prospectus, any prospectus supplement and any related free writing prospectus. We have not authorized any other person to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus is not an offer to sell securities, and it is not soliciting an offer to buy securities, in any jurisdiction where the offer or sale is not permitted. You should assume that the information appearing in this prospectus, any prospectus supplement, any related free writing prospectus as well as information we have previously filed with the SEC and incorporated by reference, is accurate as of the date of those documents only. Our business, financial condition, results of operations and prospects may have changed since those dates. This prospectus incorporates by reference, and any prospectus supplement or free writing prospectus may contain and incorporate by reference, market data and industry statistics and forecasts that are based on independent industry publications and other publicly available information. Although we believe these sources are reliable, we do not guarantee the accuracy or completeness of this information and we have not independently verified this information. Although we are not aware of any misstatements regarding the market and industry data presented in this prospectus and the documents incorporated herein by reference, these estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading “Risk Factors” contained in this prospectus, the applicable prospectus supplement and any applicable free writing prospectus, and under similar headings in other documents that are incorporated by reference into this prospectus. Accordingly, investors should not place undue reliance on this information.
We may sell securities through underwriters or dealers, through agents, directly to purchasers or through any combination of these methods. We and our agents reserve the sole right to accept or reject in whole or in part any proposed purchase of securities. The prospectus supplement, which we will prepare and file with the SEC each time we offer securities, will set forth the names of any underwriters, agents or others involved in the sale of securities, and any applicable fee, commission or discount arrangements with them. See “Plan of Distribution.”
In this prospectus, unless otherwise indicated, the “Registrant,” “our company,” “we,” “us” or “our” refer to PharmaCyte Biotech, Inc., a Nevada corporation and its consolidated subsidiaries.
We license the copyright for Cell-in-a-Box® in the United States from Austrianova Singapore Pte. Ltd. This prospectus contains references to our copyright. Solely for convenience, copyrights and trade names referred to in this prospectus, including logos, artwork and other visual displays, may appear without the ® or TM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names or trademarks to imply a relationship with, or endorsement or sponsorship of us by, any other company.
| 1 |
This prospectus summary highlights certain information about our company and other information contained elsewhere in this prospectus or in documents incorporated by reference. This summary does not contain all of the information that you should consider before making an investment decision. You should carefully read the entire prospectus, any prospectus supplement, including the section entitled “Risk Factors”, and the documents incorporated by reference into this prospectus, before making an investment decision.
This prospectus is part of a Registration Statement that we filed with the SEC utilizing a shelf registration process. Under this shelf registration process, we may sell any combination of:
| · | common stock; |
| · | preferred stock; |
| · | debt securities, in one or more series; |
| · | warrants to purchase any of the securities listed above; |
| · | rights to purchase common stock, preferred stock or warrants; and/or |
| · | units consisting of one or more of the foregoing |
in one or more offerings up to a total dollar amount of $100,000,000.00. This prospectus provides you with a general description of the securities we may offer. Each time we sell securities, we will provide a prospectus supplement that will contain specific information about the terms of that specific offering and include a discussion of any risk factors or other special considerations that apply to those securities. The prospectus supplement may also add, update or change information contained in this prospectus. You should read both this prospectus and any prospectus supplement together with the additional information described under the heading “Where You Can Find More Information.”
We are a biotechnology company focused on developing cellular therapies for cancer and diabetes based upon a proprietary cellulose-based live cell encapsulation technology known as “Cell-in-a-Box®.” The Cell-in-a-Box® technology is intended to be used as a platform upon which therapies for several types of cancer, including locally advanced, inoperable, pancreatic cancer (“LAPC”), and Type 1 and insulin dependent Type 2 diabetes will be developed. The current generation of our product candidate is referred to as “CypCaps™”. On September 1, 2020, we submitted an Investigational New Drug Application (“IND” to the U.S. Food and Drug Administration (“FDA”) for a planned Phase 2b clinical trial in LAPC, and on October 1, 2020, the Company received notice that the FDA had placed the IND on clinical hold. See “Clinical Hold.”
We are developing therapies for pancreatic and other solid cancerous tumors by using genetically engineered live human cells that we believe are capable of converting a cancer prodrug into its cancer-killing form, encapsulating those cells using the Cell-in-a-Box® technology and placing those capsules in the body as close as possible to the tumor. We believe that when the cancer prodrug is administered to a patient with a particular type of cancer that may be affected by the prodrug, the killing of the patient’s tumor may be optimized.
We are also examining ways to exploit the benefits of the Cell-in-a-Box® technology to develop therapies for cancer that involve prodrugs based upon certain constituents of the Cannabis plant; these constituents are of the class of compounds known as “cannabinoids.” Until the FDA allows us to commence the clinical trial involving LAPC described in our IND for which the FDA has placed a clinical hold, we are not spending any further resources developing this program.
| 2 |
In addition, we are developing a therapy to delay the production and accumulation of malignant ascites fluid that results from many types of abdominal cancerous tumors. Malignant ascites fluid is secreted by abdominal cancerous tumors into the abdomen after the tumors have reached a certain stage of growth. This fluid contains cancer cells that can seed and form new tumors throughout the abdomen. This fluid accumulates in the abdominal cavity, causing swelling of the abdomen, severe breathing difficulties and extreme pain.
We are using our therapy for pancreatic cancer to determine if it can prevent or delay the production and accumulation of malignant ascites fluid. As with our Cannabis program, until the FDA allows us to commence the clinical trial involving LAPC described in its IND for which the FDA has placed a clinical hold, we are not spending any further resources developing this program.
We are also developing a therapy for Type 1 diabetes and insulin-dependent Type 2 diabetes. Our diabetes therapy consists of encapsulated genetically modified insulin-producing cells. The encapsulation will be done using the Cell-in-a-Box® technology. Implanting these cells in the body is designed to function as a bio-artificial pancreas for purposes of insulin production. As with the two previous programs, we not spending any further resources developing this program until the FDA allows us to commence the clinical trial involving LAPC described in its IND for which the FDA has placed a clinical hold.
Clinical Hold
On September 1, 2020, the Company submitted an IND to the FDA for a planned Phase 2b clinical trial in LAPC. Shortly thereafter, the Company received Information Requests from the FDA related to the IND. The Company timely responded to all information requests.
On October 1, 2020, the Company received notice that the FDA had placed the IND on clinical hold.
On October 30, 2020, the FDA sent a letter to the Company setting forth the reasons for the clinical hold and specific guidance on what the Company must do to have the clinical hold lifted.
In order to address the clinical hold, the FDA has requested that we:
| · | Provide additional sequencing data and genetic stability studies; |
| · | Conduct a stability study on the final formulated drug product as well as the cells from the Master Cell Bank; |
| · | Evaluate the compatibility of the delivery devices (i.e., the prefilled syringe and microcatheter) with our drug product; |
| · | Provide additional detailed description of the manufacturing process; |
| · | Provide additional product release specifications for the encapsulated cells; |
| · | Demonstrate comparability between the 1st and 2nd generation products and ensure adequate and consistent product performance and safety between the two generations of product; |
| · | Conduct a biocompatibility assessment using the final finished capsules after the entire drug product manufacturing process (but without cells); |
| · | Address insufficiencies in Chemistry, Manufacturing and Controls information in the cross-referenced Drug Master File; |
| · | Conduct an additional nonclinical study to assess the safety, activity and distribution of the drug product; |
| · | Revise the Investigators Brochure to include any additional preclinical studies conducted in response to the clinical hold and remove any statements not supported by the data; and |
| · | Provide data from a new and extensive pig study. |
| 3 |
The FDA also requested that we address several issues not related to the clinical hold in an amendment to the IND, including:
| · | Providing a Certificate of Analysis for pc3/2B1 plasmid that includes tests for assessing purity, safety, and potency; |
| · | Performing qualification studies for the drug substance filling step to ensure that the product remains sterile and stable during the filling process; |
| · | Submitting an updated batch analysis for the drug product for the specific lot that will be used for manufacturing all future drug product; |
| · | Providing additional details for the methodology for the Resorufin (CYP2B1) potency and the PrestoBlue cell metabolic assays; |
| · | Providing a few examples of common microcatheters that fit the specifications in the Angiography Procedure Manual; |
| · | Clarifying the language in the Pharmacy Manual regarding proper use of the syringe fill with the drug product; and |
| · | Providing a discussion with data for the potential for cellular and humoral immune reactivity against the heterologous rat CYP2B1 protein and potential for induction of autoimmune-mediated toxicities in the Company’s study population. |
Since October 30, 2020, there has been no further communication with the FDA regarding the clinical hold.
We have assembled a scientific team to address the FDA requests related to the clinical hold. That team is working to complete the items requested by the FDA. We have not yet determined the estimated cost or the time necessary to complete these items. The cost and time associated with completing these items will be significant.
Among other things, we completed a 12-month product stability study, commenced physical parameter testing for CypCaps™ and commenced additional studies for the sequence of DNA encoding of its encapsulated cells. We also designed the biocompatibility tests for cytotoxicity, sensitization, irritation, acute systemic toxicity, material-mediated pyrogenicity, subacute/subchronic toxicity, genotoxicity and implantation. In addition, we have begun a compression and swelling study of CypCaps™, designed a study to determine if CypCaps™ are adversely affected by contrast medium and designed a study to show the catheters used to implant CypCaps™ do not adversely impact the encapsulated cells.
Impact of the COVID-19 Pandemic on our Operations
The coronavirus SARS-Cov2 pandemic (“COVID-19”) is causing significant, industry-wide delays in clinical trials. Although we are not yet in a clinical trial, we have filed an IND with the FDA to commence a clinical trial in LAPC. While the IND has been placed on clinical hold by the FDA, we have assessed the impact of COVID-19 on our operations. Currently, many clinical trials are being delayed due to COVID-19. There are numerous reasons for these delays. For example, patients have shown a reluctance to enroll or continue in a clinical trial due to fear of exposure to COVID-19 when they are in a hospital or doctor’s office. There are local, regional and state-wide orders and regulations restricting usual normal activity by people. These discourage and interfere with patient visits to a doctor’s office if the visit is not COVID-19 related. Healthcare providers and health systems are shifting their resources away from clinical trials toward the care of COVID-19 patients. The FDA and other healthcare providers are making product candidates for the treatment of COVID-19 a priority over product candidates unrelated to COVID-19. As of the date of this prospectus, the COVID-19 pandemic has had an impact upon our operations, although we believe that impact is not material. The impact primarily relates to delays in tasks associated with the preparation of our responses to the clinical hold, including all requested preclinical studies. There may be further delays in generating responses to the requests from the FDA related to the clinical hold.
As a result of the COVID-19 pandemic, commencement of our planned clinical trial to treat LAPC may be delayed beyond the lifting of the clinical hold should that occur. Also, enrollment may be difficult for the reasons discussed above. In addition, after enrollment in the trial, if patients contract COVID-19 during their participation in the trial or are subject to isolation or shelter in place restrictions, this may cause them to drop out of our clinical trial, miss scheduled therapy appointments or follow-up visits or otherwise fail to follow the clinical trial protocol. If patients are unable to follow the clinical trial protocol or if the trial results are otherwise affected by the consequences of the COVID-19 pandemic on patient participation or actions taken to mitigate COVID-19 spread, the integrity of data from the clinical trial may be compromised or not be accepted by the FDA. This could further adversely impact or delay our clinical development program.
| 4 |
It is highly speculative in projecting the effects of COVID-19 on our clinical development program and us generally. The effects of COVID-19 quickly and dramatically change over time. Its evolution is difficult to predict, and no one is able to say with certainty when the pandemic will subside.
COVID-19 could materially affect our operations, as well as the business or operations of third parties with whom we conduct business. Our business could be adversely affected by the effects of other future health pandemics in regions where we or third parties on which we rely have significant business operations.
Corporate History
We were incorporated in 1996. In 2013, we restructured our operations to focus on biotechnology, having been a nutraceutical products company before then. The restructuring occurred so we could develop a unique, effective and safe way to treat cancer and diabetes. On January 6, 2015, we changed our name from “Nuvilex, Inc.” to “PharmaCyte Biotech, Inc.” to reflect the nature of our business.
The address of our corporate headquarters is 23046 Avenida de la Carlota, Suite 600, Laguna Hills, CA 92653 and our telephone number is (917) 595-2850. We maintain a website at www.pharmacyte.com where general information about us is available. The information on, or that may be accessed through, our website is not incorporated by reference into and should not be considered a part of this registration statement.
To date, we have had a limited operating history with our current business model and have not produced any revenues.
| 5 |
Investing in our securities involves a high degree of risk. Prior to making a decision about investing in our securities, you should carefully consider and evaluate the specific factors discussed under the heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended April 30, 2020 filed on August 13, 2020, with the SEC, as amended, and any updates described in subsequent Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Periodic Reports on Form 8-K, all of which are incorporated herein by reference, and may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. The risks and uncertainties we have described are not the only risks that we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our operations. The occurrence of these known or unknown risks might cause you to lose all or part of your investment.
See also the statements contained under the heading “Forward-Looking Statements.”
| 6 |
This prospectus and the documents incorporated by reference herein contain “forward-looking statements” within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended ( “Exchange Act”), that are intended to qualify for the “safe harbor” created by those sections. In addition, we may make forward-looking statements in other documents filed with or furnished to the SEC, and our management and other representatives may make forward-looking statements orally or in writing to analysts, investors, representatives of the media and others. Forward-looking statements can generally be identified by the fact that they do not relate strictly to historical or current facts and include, but are not limited to, statements using terminology such as “can”, “may”, “could”, “should”, “assume”, “forecasts”, “believe”, “designated to”, “will”, “expect”, “plan”, “anticipate”, “estimate”, “potential”, “position”, “predicts”, “strategy”, “guidance”, “intend”, “budget”, “seek”, “project” or “continue”, or the negative thereof or other comparable terminology regarding beliefs, plans, expectations or intentions regarding the future, including risks relating to the recent outbreak of the coronavirus (COVID-19). You should read statements that contain these words carefully because they:
| · | discuss our future expectations; |
| · | contain projections of our future results of operations or of our financial condition; and |
| · | state other “forward-looking” information. |
We believe it is important to communicate our expectations. However, forward-looking statements are based on our current expectations, assumptions, estimates and projections about our business and our industry and are subject to known and unknown risks, uncertainties and other factors. Accordingly, our actual results and the timing of certain events may differ materially from those expressed or implied in such forward-looking statements due to a variety of factors and risks, including, but not limited to, those set forth under “Risk Factors” and “Our Company” set forth in this prospectus and the documents incorporated herein by reference, including among others, our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; whether the FDA approves our IND and lifts its clinical hold so that we can commence our planned clinical trial involving LPAC; there is substantial doubt about our ability to continue as a going concern; the success and timing of our preclinical studies and clinical trials; the potential that results of preclinical studies and clinical trials may indicate that any of our technologies and product candidates are unsafe or ineffective; our dependence on third parties in the conduct of our preclinical studies and clinical trials; the difficulties and expenses associated with obtaining and maintaining regulatory approval of our product candidates; the material adverse impact that the coronavirus pandemic may have on our business, including our planned clinical trial involving LAPC, which could materially affect our operations as well as the business or operations of third parties with whom we conduct business; and whether the FDA will approve our product candidates after our clinical trials are completed, assuming the FDA allows our clinical trial to proceed for LAPC.
All forward-looking statements and risk factors included in this prospectus are made as of the date hereof, and all forward-looking statements and risk factors included in documents incorporated herein by reference are made as of their original date, in each case based on information available to us as of the date hereof, or in the case of documents incorporated by reference, the original date of any such document, and we assume no obligations to update any forward-looking statement or risk factor, unless we are required to do so by law. If we do update one or more forward-looking statements, no inference should be drawn that we will make updates with respect to other forward-looking statements or that we will make any further updates to those forward-looking statements at any future time.
Forward-looking statements may include our plans and objectives for future operations, including plans and objectives relating to our products and our future economic performance, projections, business strategy and timing and likelihood of success. Assumptions relating to the foregoing involve judgments with respect to, among other things, future economic, competitive and market conditions, future business decisions, and the time and money required to successfully complete development and commercialization of our technologies, all of which are difficult or impossible to predict accurately and many of which are beyond our control.
Any of the assumptions underlying the forward-looking statements contained in this prospectus could prove inaccurate and, therefore, we cannot assure you that any of the results or events contemplated in any of such forward-looking statements will be realized. Based on the significant uncertainties inherent in these forward-looking statements, the inclusion of any such statement should not be regarded as a representation or as a guarantee by us that our objectives or plans will be achieved, and we caution you against relying on any of the forward looking-statements contained herein.
| 7 |
Except as otherwise provided in the applicable prospectus supplement, we intend to use the net proceeds from the sale of the securities covered by this prospectus for general corporate purposes, which may include, but are not limited to, working capital, capital expenditures, clinical trials, business development and research and development expenditures and acquisitions of new technologies or businesses. The precise amount, use and timing of the application of such proceeds will depend upon our funding requirements and the availability and cost of other capital. Additional information on the use of net proceeds from an offering of securities covered by this prospectus may be set forth in the prospectus supplement relating to the specific offering.
| 8 |
We have never declared or paid any cash dividends on our common
stock. We do not anticipate paying any cash dividends to stockholders in the foreseeable future. In addition, any future determination
to pay cash dividends will be at the discretion of our Board of Directors (“Board”) and will be dependent upon our
financial condition, results of operations, capital requirements, and such other factors as our Board deems relevant.
| 9 |
DESCRIPTIONS OF THE SECURITIES WE MAY OFFER
The descriptions of the securities contained in this prospectus, together with any applicable prospectus supplement, summarize all the material terms and provisions of the various types of securities that we may offer. We will describe in the applicable prospectus supplement relating to a particular offering the specific terms of the securities offered by that prospectus supplement. We will indicate in the applicable prospectus supplement if the terms of the securities differ from the terms we have summarized below. We will also include in the prospectus supplement information, where applicable, regarding material United States federal income tax considerations relating to the securities.
We may sell from time to time, in one or more offerings:
| · | shares of our common stock; |
| · | shares of our preferred stock; |
| · | debt securities; |
| · | warrants to purchase any of the securities listed above; |
| · | rights to purchase common stock, preferred stock or warrants; and/or |
| · | units consisting of one or more of the foregoing. |
This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement.
Capital Stock
General
The following description of common stock and preferred stock, together with the additional information we include in any applicable prospectus supplement, summarizes the material terms and provisions of the common stock and preferred stock that we may offer under this prospectus but is not complete. For the complete terms of our common stock and preferred stock, please refer to our articles of incorporation, as may be amended from time to time (“Articles of Incorporation”), any certificates of designation for our preferred stock, that may be authorized from time to time, and our bylaws, as amended from time to time. Nevada Law may also affect the terms of these securities. While the terms we have summarized below will apply generally to any future common stock or preferred stock that we may offer, we will describe the specific terms of any series of these securities in more detail in the applicable prospectus supplement. If we so indicate in a prospectus supplement, the terms of any common stock or preferred stock we offer under that prospectus supplement may differ from the terms we describe below.
As of April 1, 2021, our authorized capital stock consists of 2,490,000,000 shares of common stock, par value $0.0001 per share, of which 2,341,410,405 shares were issued and outstanding, and 10,000,000 shares of preferred stock, par value $0.0001 per share, of which no shares were issued and outstanding. The actual number of stockholders is greater than the number of stockholders of record and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees. This number of holders of record also does not include stockholders whose shares may be held in trust by other entities. In addition, as of April 1, 2021, there were issued and outstanding options to purchase 62,600,000 shares of our common stock and warrants to purchase 4,472,129 shares of our common stock. The authorized and unissued shares of common stock and preferred stock are available for issuance without further action by our stockholders, unless such action is required by applicable law or the rules of any stock exchange on which our securities may be listed. Unless approval of our stockholders is so required, our Board will not seek stockholder approval for the issuance and sale of our common stock.
| 10 |
Common Stock
Holders of our common stock are entitled to one vote for each share issued and outstanding held on all matters to be voted upon by the stockholders. Our shares of common stock have no preemptive, conversion, or redemption rights. The rights, preferences, and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock we may issue in the future. Upon the sale of substantially all of our stock or assets or dissolution, liquidation or winding up, and after all liquidation preferences payable to any series of preferred stock entitled thereto have been satisfied, our remaining assets shall be distributed to all holders of common stock and any similarly situated stockholders who are not entitled to any liquidation preference or, if there be an insufficient amount to pay all such stockholders, then ratably among such holders. All of our issued and outstanding shares of common stock are fully paid and non-assessable. The holders of shares of our common stock will be entitled to such dividends and other distributions in cash, stock or property from our assets or funds legally available for such purposes as may be declared from time to time by our Board.
Our common stock is quoted on OTCQB under the symbol “PMCB.” American Stock Transfer & Trust Company is the transfer agent and registrar for our common stock.
Preferred Stock
Our Articles of Incorporation provides that our Board may, by resolution, designate classes of preferred stock in the future. The designated series of preferred stock will have such powers, designations, preferences and relative participation or optional or other special rights and qualifications, limitations or restrictions as expressed in the related resolution adopted by the Board. Once designated by our Board, each series of preferred stock will have specific financial and other terms that will be described in a prospectus supplement. The description of the preferred stock that is set forth in any prospectus supplement is not complete without reference to the documents that govern the preferred stock. These include our Articles of Incorporation and any certificates of designation that our Board may adopt. Before the issuance of shares of each series of preferred stock, the Board is required by the Nevada Revised Statutes and our Articles of Incorporation to adopt resolutions and file a certificate of designations with the Secretary of State of the State of Nevada. The related certificate of designation fixes for each class or series the designations, powers, preferences, rights, qualifications, limitations and restrictions, including, but not limited to, some or all of the following:
| · | the number of shares constituting that series and the distinctive designation of that series, which number may be increased or decreased (but not below the number of shares then outstanding) from time to time by action of the Board; |
| · | the dividend rate and the manner and frequency of payment of dividends on the shares of that series, whether dividends will be cumulative, and, if so, from which date; |
| · | whether that series will have voting rights, in addition to any voting rights provided by law, and, if so, the terms of such voting rights; |
| · | whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the Board may determine; |
| · | whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption; |
| · | whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund; |
| · | whether or not the shares of the series will have priority over or be on a parity with or be junior to the shares of any other series or class in any respect; |
| · | the rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation, and the relative rights or priority, if any, of payment of shares of that series; and |
| · | any other relative rights, preferences and limitations of that series. |
| 11 |
Although our Board has no intention at the present time of doing so, it could authorize the issuance of a series of preferred stock that could, depending on the terms of such series, impede the completion of a merger, tender offer or other takeover attempt. All shares of preferred stock offered hereby will, when issued, be fully paid and non-assessable, including shares of preferred stock issued upon the exercise of preferred stock warrants or subscription rights, if any.
We have authorized 10,000,000 shares of preferred stock, with a par value of $0.0001, of which one share has been designated as "Series A Preferred Stock" and 13,500 shares have been designated as “Series E Preferred Stock.” The one share of Series A Preferred Stock was issued on October 30, 2019 and repurchased by the Company on December 3, 2019. As of April 30, 2020, there are no shares of preferred stock issued and outstanding.
The descriptions of the Series A Preferred Stock and the Series E Preferred Stock below is qualified in its entirety by reference to the Company’s Articles of Incorporation, as amended.
Series A Convertible Preferred Stock
The Series A Preferred Stock has the following features:
| · | There is one share of preferred stock designated as Series A Preferred Stock; |
| · | The Series A Preferred Stock has a number of votes at any time equal to the number of votes then held by all other shareholders of the Company having a right to vote on any matter plus one. The Certificate of Designations that designated the terms of the Series A Preferred Stock cannot be amended without the consent of the holder of the Series A Preferred Stock; |
| · | We may redeem the Series A Preferred Stock at any time for a redemption price of $1.00 paid to the holder of the share of Series A Preferred Stock; and |
| · | The Series A Preferred Stock has no rights of transfer, conversion, dividends, preferences upon liquidation or participation in any distributions to shareholders. |
Series E Convertible Preferred Stock
The Series E Convertible Preferred Stock have the following features:
| · | The holders of Series E Convertible Preferred Stock are entitled to receive cash out of our assets before any amount is paid to the holders of any capital stock of any class junior in rank to the shares of Series E Convertible Preferred Stock; |
| · | Each share of Series E Convertible Preferred Stock is convertible, at the holder's option, into shares of common stock at the average closing bid price of the common stock for five trading days prior to the conversion date; |
| · | We have the right, in our sole discretion, at any time 110 days after issuance of shares of Series E Convertible Preferred Stock, to redeem all of the shares of Series E Convertible Preferred Stock upon thirty days advance written notice at a redemption price equal to the par value of the shares of the Series E Convertible Preferred Stock; and |
| · | At every meeting of stockholders every holder of shares of Series E Convertible Preferred Stock is entitled to 50,000 votes for each share of Series E Convertible Preferred Stock with the same and identical voting rights as a holder of a share of common stock. |
| 12 |
Anti-Takeover Effects of Nevada Law
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the NRS, generally prohibit a Nevada corporation with at least 200 stockholders of record from engaging in various “combination” transactions with any interested stockholder for a period of two years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the Board prior to the date the interested stockholder obtained such status or the combination is approved by the Board and thereafter is approved at a meeting of the stockholders by the affirmative vote of stockholders representing at least 60% of the outstanding voting power held by disinterested stockholders, and extends beyond the expiration of the two-year period, unless:
| · | the combination was approved by the Board prior to the person becoming an interested stockholder or the transaction by which the person first became an interested stockholder was approved by the Board before the person became an interested stockholder or the combination is later approved by a majority of the voting power held by disinterested stockholders; or |
| · | if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the two years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
A “combination” is generally defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer, or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, (c) 10% or more of the earning power or net income of the corporation, and (d) certain other transactions with an interested stockholder or an affiliate or associate of an interested stockholder.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within two years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire us even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
The provisions of Nevada law could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the market price of our common stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in the composition of our Board and management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
In addition, the NRS provides for statutes, Sections 78.378 to 78.3793, inclusive, of the NRS, that limit the voting rights of the acquisition of a “controlling interest” defined to occur at three ownership thresholds of one-fifth, one-third and a majority of the corporation’s voting power. Although our Articles of Incorporation have not opted out of these statutes, the limitations on voting rights apply only to a corporation with 200 or more stockholders of record, at least 100 of whom have addresses in the State of Nevada appearing on the corporation’s stock ledger during the 90 days immediately preceding the date of the acquisition.
| 13 |
Warrants
The following description, together with the additional information we may include in any applicable prospectus supplement or free writing prospectus, summarizes the material terms and provisions of the warrants that we may offer under this prospectus and any related warrant agreement and warrant certificate. While the terms summarized below will apply generally to any warrants that we may offer, we will describe the specific terms of any series of warrants in more detail in the applicable prospectus supplement. If we indicate in the prospectus supplement, the terms of any warrants offered under that prospectus supplement may differ from the terms described below. Specific warrant agreements will contain additional important terms and provisions and will be incorporated by reference as an exhibit to the Registration Statement which includes this prospectus.
General
We may issue warrants for the purchase of common stock, preferred stock and/or debt securities in one or more series. We may issue warrants independently or together with common stock, preferred stock and/or debt securities, and the warrants may be attached to or separate from these securities.
We will evidence each series of warrants by warrant certificates that we may issue under a separate agreement. We may enter into a warrant agreement with a warrant agent. Each warrant agent may be a bank or trust company that we select which has its principal office in the United States. We may also choose to act as our own warrant agent. We will indicate the name and address of any such warrant agent in the applicable prospectus supplement relating to a particular series of warrants.
We will describe in the applicable prospectus supplement the terms of the series of warrants, including:
| · | the offering price and aggregate number of warrants offered; |
| · | if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; |
| · | if applicable, the date on and after which the warrants and the related securities will be separately transferable; |
| · | in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at, and currency in which, this principal amount of debt securities may be purchased upon such exercise; |
| · | in the case of warrants to purchase common stock or preferred stock, the number or amount of shares of common stock or preferred stock, as the case may be, purchasable upon the exercise of one warrant and the price at which and currency in which these shares may be purchased upon such exercise; |
| · | the manner of exercise of the warrants, including any cashless exercise rights; |
| · | the warrant agreement under which the warrants will be issued; |
| · | the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreement and the warrants; |
| · | anti-dilution provisions of the warrants, if any; |
| · | the terms of any rights to redeem or call the warrants; |
| · | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; |
| · | the dates on which the right to exercise the warrants will commence and expire or, if the warrants are not continuously exercisable during that period, the specific date or dates on which the warrants will be exercisable; |
| · | the manner in which the warrant agreement and warrants may be modified; |
| · | the identities of the warrant agent and any calculation or other agent for the warrants; |
| · | federal income tax consequences of holding or exercising the warrants; |
| · | the terms of the securities issuable upon exercise of the warrants; |
| · | any securities exchange or quotation system on which the warrants or any securities deliverable upon exercise of the warrants may be listed or quoted; and |
| · | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
| 14 |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
| · | in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; or |
| · | in the case of warrants to purchase common stock or preferred stock, the right to receive dividends, if any, or, payments upon our liquidation, dissolution or winding up or to exercise voting rights, if any. |
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to 5:00 P.M. eastern time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information and paying the required exercise price by the methods provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate, and in the applicable prospectus supplement, the information that the holder of the warrant will be required to deliver to the warrant agent.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for the remaining amount of warrants.
Enforceability of Rights by Holders of Warrants
Any warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action the holder’s right to exercise, and receive the securities purchasable upon exercise of, its warrants in accordance with their terms.
Warrant Agreement Will Not Be Qualified Under Trust Indenture Act
No warrant agreement will be qualified as an indenture, and no warrant agent will be required to qualify as a trustee, under the Trust Indenture Act of 1939, as amended (“Trust Indenture Act”). Therefore, holders of warrants issued under a warrant agreement will not have the protection of the Trust Indenture Act with respect to their warrants.
Governing Law
Each warrant agreement and any warrants issued under the warrant agreements will be governed by New York law.
| 15 |
Calculation Agent
Any calculations relating to warrants may be made by a calculation agent, an institution that we appoint as our agent for this purpose. The prospectus supplement for a particular warrant will name the institution that we have appointed to act as the calculation agent for that warrant as of the original issue date for that warrant, if any. We may appoint a different institution to serve as calculation agent from time to time after the original issue date without the consent or notification of the holders. The calculation agent’s determination of any amount of money payable or securities deliverable with respect to a warrant will be final and binding in the absence of manifest error.
Outstanding Warrants
As of April 1, 2021, we had outstanding four warrants to purchase 4,472,129 shares of our common stock at a weighted average exercise price of $0.039.
Debt Securities
The following description, together with the additional information we include in any applicable prospectus supplement or free writing prospectus, summarizes certain general terms and provisions of the debt securities that we may offer under this prospectus. When we offer to sell a particular series of debt securities, we will describe the specific terms of the series in a supplement to this prospectus. We will also indicate in the supplement to what extent the general terms and provisions described in this prospectus apply to a particular series of debt securities. To the extent the information contained in the prospectus supplement differs from this summary description, you should rely on the information in the prospectus supplement.
We may issue debt securities either separately, or together with, or upon the conversion or exercise of or in exchange for, other securities described in this prospectus. Debt securities may be our senior, senior subordinated or subordinated obligations and, unless otherwise specified in a supplement to this prospectus, the debt securities will be our direct, unsecured obligations and may be issued in one or more series.
The debt securities will be issued under an indenture between us and a trustee named in the prospectus supplement. We have summarized select portions of the indenture below. The summary is not complete. The form of the indenture has been filed as an exhibit to the registration statement and you should read the indenture for provisions that may be important to you. In the summary below, we have included references to the section numbers of the indenture so that you can easily locate these provisions. Capitalized terms used in the summary and not defined herein have the meanings specified in the indenture.
General
The terms of each series of debt securities will be established by or pursuant to a resolution of our Board and set forth or determined in the manner provided in a resolution of our Board, in an officer’s certificate or by a supplemental indenture. (Section 2.2) The particular terms of each series of debt securities will be described in a prospectus supplement relating to such series (including any pricing supplement or term sheet).
We can issue an unlimited amount of debt securities under the indenture that may be in one or more series with the same or various maturities, at par, at a premium, or at a discount. (Section 2.1) We will set forth in a prospectus supplement (including any pricing supplement or term sheet) relating to any series of debt securities being offered, the aggregate principal amount and the following terms of the debt securities, if applicable:
| · | the title and ranking of the debt securities (including the terms of any subordination provisions); |
| · | the price or prices (expressed as a percentage of the principal amount) at which we will sell the debt securities; |
| · | any limit on the aggregate principal amount of the debt securities; |
| · | the date or dates on which the principal of a particular series of debt securities is payable; |
| · | the rate or rates (which may be fixed or variable) per annum or the method used to determine the rate or rates (including any commodity, commodity index, stock exchange index or financial index) at which the debt securities will bear interest, the date or dates from which interest will accrue, the date or dates on which interest will commence and be payable and any regular record date for the interest payable on any interest payment date; |
| 16 |
| · | the place or places where principal of, and interest, if any, on the debt securities will be payable (and the method of such payment), where the debt securities of such series may be surrendered for registration of transfer or exchange, and where notices and demands to us in respect of the debt securities may be delivered; |
| · | the period or periods within which, the price or prices at which and the terms and conditions upon which we may redeem the debt securities; |
| · | any obligation we have to redeem or purchase the debt securities pursuant to any sinking fund or analogous provisions or at the option of a holder of debt securities and the period or periods within which, the price or prices at which and the terms and conditions upon which the debt securities of a particular series shall be redeemed or purchased, in whole or in part, pursuant to such obligation; |
| · | the dates on which and the price or prices at which we will repurchase debt securities at the option of the holders of debt securities and other detailed terms and provisions of these repurchase obligations; |
| · | the denominations in which the debt securities will be issued, if other than denominations of $1,000 and any integral multiple thereof; |
| · | whether the debt securities will be issued in the form of certificated debt securities or global debt securities; |
| · | the portion of principal amount of the debt securities payable upon declaration of acceleration of the maturity date, if other than the principal amount; |
| · | the currency of denomination of the debt securities, which may be U.S. dollars or any foreign currency, and if such currency of denomination is a composite currency, the agency or organization, if any, responsible for overseeing such composite currency; |
| · | the designation of the currency, currencies or currency units in which payment of principal of, and premium and interest on, the debt securities will be made; |
| · | if payments of principal of, or premium or interest on, the debt securities will be made in one or more currencies or currency units other than that or those in which the debt securities are denominated, the manner in which the exchange rate with respect to these payments will be determined; |
| · | the manner in which the amounts of payment of principal of, and premium, if any, and interest on, the debt securities will be determined, if these amounts may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index; |
| · | any provisions relating to any security provided for the debt securities; |
| · | any addition to, deletion of or change in the Events of Default described in this prospectus or in the indenture with respect to the debt securities and any change in the acceleration provisions described in this prospectus or in the indenture with respect to the debt securities; |
| · | any addition to, deletion of or change in the covenants described in this prospectus or in the indenture with respect to the debt securities; |
| · | any depositaries, interest rate calculation agents, exchange rate calculation agents or other agents with respect to the debt securities; |
| · | the provisions, if any, relating to conversion or exchange of any debt securities of such series, including if applicable, the conversion or exchange price and period, provisions as to whether conversion or exchange will be mandatory, the events requiring an adjustment of the conversion or exchange price and provisions affecting conversion or exchange; |
| · | any other terms of the debt securities, which may supplement, modify or delete any provision of the indenture as it applies to that series, including any terms that may be required under applicable law or regulations or advisable in connection with the marketing of the securities; and |
| · | whether any of our direct or indirect subsidiaries will guarantee the debt securities of that series, including the terms of subordination, if any, of such guarantees. (Section 2.2) |
We may issue debt securities that provide for an amount less than their stated principal amount to be due and payable upon declaration of acceleration of their maturity pursuant to the terms of the indenture. We will provide you with information on the federal income tax considerations and other special considerations applicable to any of these debt securities in the applicable prospectus supplement.
If we denominate the purchase price of any of the debt securities in a foreign currency or currencies or a foreign currency unit or units, or if the principal of, and premium, if any, and interest on, any series of debt securities is payable in a foreign currency or currencies or a foreign currency unit or units, we will provide you with information on the restrictions, elections, general tax considerations, specific terms and other information with respect to that issue of debt securities and such foreign currency or currencies or foreign currency unit or units in the applicable prospectus supplement.
| 17 |
Transfer and Exchange
Each debt security will be represented by either one or more global securities registered in the name of The Depository Trust Company (“DTC” or the “Depositary”) or a nominee of the Depositary (we will refer to any debt security represented by a global debt security as a “book-entry debt security”), or a certificate issued in definitive registered form (we will refer to any debt security represented by a certificated security as a “certificated debt security”) as set forth in the applicable prospectus supplement. Except as set forth under the heading “Global Debt Securities and Book-Entry System” below, book-entry debt securities will not be issuable in certificated form.
Certificated Debt Securities. You may transfer or exchange certificated debt securities at any office we maintain for this purpose in accordance with the terms of the indenture. (Section 2.4) No service charge will be made for any transfer or exchange of certificated debt securities, but we may require payment of a sum sufficient to cover any tax or other governmental charge payable in connection with a transfer or exchange. (Section 2.7)
You may effect the transfer of certificated debt securities and the right to receive the principal of, premium and interest on certificated debt securities only by surrendering the certificate representing those certificated debt securities and either reissuance by us or the trustee of the certificate to the new holder or the issuance by us or the trustee of a new certificate to the new holder.
Global Debt Securities and Book-Entry System. Each global debt security representing book-entry debt securities will be deposited with, or on behalf of, the Depositary, and registered in the name of the Depositary or a nominee of the Depositary. Please see the section entitled “Global Securities” for more information.
Covenants
We will set forth in the applicable prospectus supplement any restrictive covenants applicable to any issue of debt securities. (Article IV)
No Protection in the Event of a Change of Control
Unless we state otherwise in the applicable prospectus supplement, the debt securities will not contain any provisions that may afford holders of the debt securities protection in the event we have a change in control or in the event of a highly leveraged transaction (whether or not such transaction results in a change in control) which could adversely affect holders of debt securities.
Consolidation, Merger and Sale of Assets
We may not consolidate with or merge with or into, or convey, transfer or lease all or substantially all of our properties and assets to, any person (a “successor person”) unless:
| · | we are the surviving corporation or the successor person (if other than our company) is a corporation organized and validly existing under the laws of any U.S. domestic jurisdiction and expressly assumes our obligations on the debt securities and under the indenture; |
| · | immediately after giving effect to the transaction, no Default or Event of Default, shall have occurred and be continuing; and |
| · | certain other conditions are met. |
Notwithstanding the above, any of our subsidiaries may consolidate with, merge into or transfer all or part of its properties to us. (Section 5.1)
| 18 |
Events of Default
“Event of Default” means with respect to any series of debt securities, any of the following:
| · | default in the payment of any interest upon any debt security of that series when it becomes due and payable, and continuance of such default for a period of 30 days (unless the entire amount of the payment is deposited by us with the trustee or with a paying agent prior to the expiration of the 30-day period); |
| · | default in the payment of principal of any debt security of that series at its maturity; |
| · | default in the performance or breach of any other covenant or warranty by us in the indenture or any debt security (other than a covenant or warranty that has been included in the indenture solely for the benefit of a series of debt securities other than that series), which default continues uncured for a period of 60 days after we receive written notice from the trustee or our company and the trustee receive written notice from the holders of not less than 25% in principal amount of the outstanding debt securities of that series as provided in the indenture; |
| · | certain voluntary or involuntary events of bankruptcy, insolvency or reorganization of our company; or |
| · | any other Event of Default provided with respect to debt securities of that series that is described in the applicable prospectus supplement. (Section 6.1) |
No Event of Default with respect to a particular series of debt securities (except as to certain events of bankruptcy, insolvency or reorganization) necessarily constitutes an Event of Default with respect to any other series of debt securities. (Section 6.1) The occurrence of certain Events of Default or an acceleration under the indenture may constitute an event of default under certain indebtedness of ours or our subsidiaries outstanding from time to time.
We will provide the trustee written notice of any Default or Event of Default within 30 days of becoming aware of the occurrence of such Default or Event of Default, which notice will describe in reasonable detail the status of such Default or Event of Default and what action we are taking or propose to take in respect thereof. (Section 6.1)
If an Event of Default with respect to debt securities of any series at the time outstanding occurs and is continuing, then the trustee or the holders of not less than 25% in principal amount of the outstanding debt securities of that series may, by a notice in writing to us (and to the trustee if given by the holders), declare to be due and payable immediately the principal of (or, if the debt securities of that series are discount securities, that portion of the principal amount as may be specified in the terms of that series) and accrued and unpaid interest, if any, on all debt securities of that series. In the case of an Event of Default resulting from certain events of bankruptcy, insolvency or reorganization, the principal (or such specified amount) of and accrued and unpaid interest, if any, on all outstanding debt securities will become and be immediately due and payable without any declaration or other act on the part of the trustee or any holder of outstanding debt securities. At any time after a declaration of acceleration with respect to debt securities of any series has been made, but before a judgment or decree for payment of the money due has been obtained by the trustee, the holders of a majority in principal amount of the outstanding debt securities of that series may rescind and annul the acceleration if all Events of Default, other than the non-payment of accelerated principal and interest, if any, with respect to debt securities of that series, have been cured or waived as provided in the indenture. (Section 6.2) We refer you to the prospectus supplement relating to any series of debt securities that are discount securities for the particular provisions relating to acceleration of a portion of the principal amount of such discount securities upon the occurrence of an Event of Default.
The indenture provides that the trustee may refuse to perform any duty or exercise any of its rights or powers under the indenture, unless the trustee receives indemnity satisfactory to it against any cost, liability or expense that might be incurred by it in performing such duty or exercising such right or power. (Section 7.1(e)) Subject to certain rights of the trustee, the holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the trustee or exercising any trust or power conferred on the trustee with respect to the debt securities of that series. (Section 6.12)
| 19 |
No holder of any debt security of any series will have any right to institute any proceeding, judicial or otherwise, with respect to the indenture or for the appointment of a receiver or trustee, or for any remedy under the indenture, unless:
| · | that holder has previously given to the trustee written notice of a continuing Event of Default with respect to debt securities of that series; and |
| · | the holders of not less than 25% in principal amount of the outstanding debt securities of that series have made written request, and offered indemnity or security satisfactory to the trustee, to the trustee to institute the proceeding as trustee, and the trustee has not received from the holders of not less than a majority in principal amount of the outstanding debt securities of that series a direction inconsistent with that request and has failed to institute the proceeding within 60 days. (Section 6.7) |
Notwithstanding any other provision in the indenture, the holder of any debt security will have an absolute and unconditional right to receive payment of the principal of, and premium and any interest on, that debt security on or after the due dates expressed in that debt security and to institute suit for the enforcement of payment. (Section 6.8).
The indenture requires us, within 120 days after the end of our fiscal year, to furnish to the trustee a statement as to compliance with the indenture. (Section 4.3) If a Default or Event of Default occurs and is continuing with respect to the securities of any series and if it is known to a responsible officer of the trustee, the trustee shall mail to each holder of the securities of that series notice of a Default or Event of Default within 90 days after it occurs or, if later, after a responsible officer of the trustee has knowledge of such Default or Event of Default. The indenture provides that the trustee may withhold notice to the holders of debt securities of any series of any Default or Event of Default (except in payment on any debt securities of that series) with respect to debt securities of that series if the trustee determines in good faith that withholding notice is in the interest of the holders of those debt securities. (Section 7.5)
Modification and Waiver
We and the trustee may modify, amend or supplement the indenture or the debt securities of any series without the consent of any holder of any debt security:
| · | to cure any ambiguity, defect or inconsistency; |
| · | to comply with covenants in the indenture described above under the heading “Consolidation, Merger and Sale of Assets”; |
| · | to provide for uncertificated securities in addition to or in place of certificated securities; |
| · | to add guarantees with respect to debt securities of any series or secure debt securities of any series; |
| · | to surrender any of our rights or powers under the indenture; |
| · | to add covenants or Events of Default for the benefit of the holders of debt securities of any series; |
| · | to comply with the applicable procedures of the applicable depositary; |
| · | to make any change that does not adversely affect the rights of any holder of debt securities; |
| · | to provide for the issuance of and establish the form and terms and conditions of debt securities of any series as permitted by the indenture; |
| · | to effect the appointment of a successor trustee with respect to the debt securities of any series and to add to or change any of the provisions of the indenture to provide for or facilitate administration by more than one trustee; or |
| · | to comply with requirements of the SEC in order to effect or maintain the qualification of the indenture under the Trust Indenture Act. (Section 9.1) |
| 20 |
We may also modify and amend the indenture with the consent of the holders of at least a majority in principal amount of the outstanding debt securities of each series affected by the modifications or amendments. We may not make any modification or amendment without the consent of the holders of each affected debt security then outstanding if that amendment will:
| · | reduce the amount of debt securities whose holders must consent to an amendment, supplement or waiver; |
| · | reduce the rate of or extend the time for payment of interest (including default interest) on any debt security; |
| · | reduce the principal of or premium on or change the fixed maturity of any debt security or reduce the amount of, or postpone the date fixed for, the payment of any sinking fund or analogous obligation with respect to any series of debt securities; |
| · | reduce the principal amount of discount securities payable upon acceleration of maturity; |
| · | waive a Default or Event of Default in the payment of the principal of, or premium or interest on, any debt security (except a rescission of acceleration of the debt securities of any series by the holders of at least a majority in aggregate principal amount of the then outstanding debt securities of that series and a waiver of the payment default that resulted from such acceleration); |
| · | make the principal of, or premium or interest on, any debt security payable in currency other than that stated in the debt security; |
| · | make any change to certain provisions of the indenture relating to, among other things, the right of holders of debt securities to receive payment of the principal of, and premium and interest on, those debt securities and to institute suit for the enforcement of any such payment and to waivers or amendments; or |
| · | waive a redemption payment with respect to any debt security. (Section 9.3) |
Except for certain specified provisions, the holders of at least a majority in principal amount of the outstanding debt securities of any series may on behalf of the holders of all debt securities of that series waive our compliance with provisions of the indenture. (Section 9.2) The holders of a majority in principal amount of the outstanding debt securities of any series may on behalf of the holders of all the debt securities of such series waive any past default under the indenture with respect to that series and its consequences, except a default in the payment of the principal of, or any interest on, any debt security of that series; provided, however, that the holders of a majority in principal amount of the outstanding debt securities of any series may rescind an acceleration and its consequences, including any related payment default that resulted from the acceleration. (Section 6.13)
Defeasance of Debt Securities and Certain Covenants in Certain Circumstances
Legal Defeasance. The indenture provides that, unless otherwise provided by the terms of the applicable series of debt securities, we may be discharged from any and all obligations in respect of the debt securities of any series (subject to certain exceptions). We will be so discharged upon the deposit with the trustee, in trust, of money and/or U.S. government obligations or, in the case of debt securities denominated in a single currency other than U.S. dollars, government obligations of the government that issued or caused to be issued such currency, that, through the payment of interest and principal in accordance with their terms, will provide money or U.S. government obligations in an amount sufficient in the opinion of a nationally recognized firm of independent public accountants or investment bank to pay and discharge each installment of principal of, premium and interest on, and any mandatory sinking fund payments in respect of, the debt securities of that series on the stated maturity of those payments in accordance with the terms of the indenture and those debt securities.
This discharge may occur only if, among other things, we have delivered to the trustee an opinion of counsel stating that we have received from, or there has been published by, the U.S. Internal Revenue Service a ruling or, since the date of execution of the indenture, there has been a change in the applicable U.S. federal income tax law, in either case to the effect that, and based thereon such opinion shall confirm that, the holders of the debt securities of that series will not recognize income, gain or loss for U.S. federal income tax purposes as a result of the deposit, defeasance and discharge and will be subject to U.S. federal income tax on the same amounts and in the same manner and at the same times as would have been the case if the deposit, defeasance and discharge had not occurred. (Section 8.3)
| 21 |
Defeasance of Certain Covenants. The indenture provides that, unless otherwise provided by the terms of the applicable series of debt securities, upon compliance with certain conditions:
| · | we may omit to comply with the covenant described under the heading “Consolidation, Merger and Sale of Assets” and certain other covenants set forth in the indenture, as well as any additional covenants that may be set forth in the applicable prospectus supplement; and |
| · | any omission to comply with those covenants will not constitute a Default or an Event of Default with respect to the debt securities of that series (“covenant defeasance”). |
The conditions include:
| · | depositing with the trustee money and/or U.S. government obligations or, in the case of debt securities denominated in a single currency other than U.S. dollars, government obligations of the government that issued or caused to be issued such currency, that, through the payment of interest and principal in accordance with their terms, will provide money in an amount sufficient in the opinion of a nationally recognized firm of independent public accountants or investment bank to pay and discharge each installment of principal of, premium and interest on, and any mandatory sinking fund payments in respect of, the debt securities of that series on the stated maturity of those payments in accordance with the terms of the indenture and those debt securities; and |
| · | delivering to the trustee an opinion of counsel to the effect that the holders of the debt securities of that series will not recognize income, gain or loss for U.S. federal income tax purposes as a result of the deposit and related covenant defeasance and will be subject to U.S. federal income tax on the same amounts and in the same manner and at the same times as would have been the case if the deposit and related covenant defeasance had not occurred. (Section 8.4) |
No Personal Liability of Directors, Officers, Employees or Securityholders
None of our past, present or future directors, officers, employees or securityholders, as such, will have any liability for any of our obligations under the debt securities or the indenture or for any claim based on, or in respect or by reason of, such obligations or their creation. By accepting a debt security, each holder waives and releases all such liability. This waiver and release is part of the consideration for the issue of the debt securities. However, this waiver and release may not be effective to waive liabilities under U.S. federal securities laws, and it is the view of the SEC that such a waiver is against public policy.
Governing Law
The indenture and the debt securities, including any claim or controversy arising out of or relating to the indenture or the debt securities, will be governed by the laws of the State of New York.
The indenture will provide that we, the trustee and the holders of the debt securities (by their acceptance of the debt securities) irrevocably waive, to the fullest extent permitted by applicable law, any and all right to trial by jury in any legal proceeding arising out of or relating to the indenture, the debt securities or the transactions contemplated thereby.
| 22 |
The indenture will provide that any legal lawsuit, action or proceeding arising out of or based upon the indenture or the transactions contemplated thereby may be instituted in the federal courts of the United States of America located in the City of New York or the courts of the State of New York in each case located in the City of New York, and we, the trustee and the holder of the debt securities (by their acceptance of the debt securities) irrevocably submit to the non-exclusive jurisdiction of such courts in any such suit, action or proceeding. The indenture will further provide that service of any process, summons, notice or document by mail (to the extent allowed under any applicable statute or rule of court) to such party’s address set forth in the indenture will be effective service of process for any suit, action or other proceeding brought in any such court. The indenture will further provide that we, the trustee and the holders of the debt securities (by their acceptance of the debt securities) irrevocably and unconditionally waive any objection to the laying of venue of any suit, action or other proceeding in the courts specified above and irrevocably and unconditionally waive and agree not to plead or claim any such suit, action or other proceeding has been brought in an inconvenient forum. (Section 10.10)
Rights
We may issue rights to purchase common stock, preferred stock or warrants that we may offer to our security holders in one or more series. The rights may or may not be transferable by the persons purchasing or receiving the rights. In connection with any rights offering, we may enter into a standby underwriting or other arrangement with one or more underwriters or other persons pursuant to which such underwriters or other persons would purchase any offered securities remaining unsubscribed for after such rights offering. Each series of rights will be issued under a separate rights agent agreement to be entered into between us and a bank or trust company, as rights agent, that we will name in the applicable prospectus supplement. The rights agent will act solely as our agent in connection with the rights and will not assume any obligation or relationship of agency or trust for or with any holders of rights certificates or beneficial owners of rights. A copy of the form of rights agent or subscription agent agreement, including the form of rights certificate representing a series of rights, will be filed with the SEC in connection with the offering of a particular series of rights.
The prospectus supplement relating to any rights that we offer will include specific terms relating to the offering, including, among other matters:
| · | the title of the rights; |
| · | the securities for which the rights are exercisable; |
| · | the date of determining the security holders entitled to the rights distribution; |
| · | the aggregate number of rights issued and the aggregate number of shares of common stock or preferred stock or warrants purchasable upon exercise of the rights; |
| · | the extent to which the rights are transferable; |
| · | the exercise price; |
| · | any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the rights; |
| · | the conditions to completion of the rights offering; |
| · | any applicable federal income tax considerations; |
| · | if applicable, the material terms of any standby underwriting or other purchase arrangement that we may enter into in connection with the rights offering; |
| · | the date on which the right to exercise the rights will commence and the date on which the rights will expire; and |
| · | any other terms of the rights, including terms, procedures and limitations relating to the exchange and exercise of the rights. |
Each right would entitle the holder of the rights to purchase for cash the amount of shares of common stock or preferred stock or warrants at the exercise price set forth in the applicable prospectus supplement. Rights may be exercised at any time up to the close of business on the expiration date for the rights provided in the applicable prospectus supplement. After the close of business on the expiration date, all unexercised rights will become void.
| 23 |
We may determine to offer any unsubscribed securities directly to persons other than our security holders, to or through agents, underwriters or dealers or through a combination of such methods, including pursuant to standby arrangements, as described in the applicable prospectus supplement.
Until a holder exercises the rights to purchase shares of our common stock or preferred stock or warrants, the holder will not have any rights as a holder of shares of our common stock or preferred stock or warrants, as the case may be, by virtue of ownership of the rights.
Units
We may issue units consisting of one or more of the other securities described in this prospectus, in any prospectus supplement or a free writing prospectus in any combination. Each unit will be issued so that the holder of the unit is also the holder, with the rights and obligations of a holder, of each security included in the unit. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date or upon the occurrence of a specified event or occurrence.
The applicable prospectus supplement or free writing prospectus will describe:
| · | the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; |
| · | any unit agreement under which the units will be issued; |
| · | any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units; and |
| · | whether the units will be issued in fully registered or global form. |
Global Securities
Book-Entry, Delivery and Form
Unless we indicate differently in any applicable prospectus supplement or free writing prospectus, the securities initially will be issued in book-entry form and represented by one or more global notes or global securities (collectively, “global securities”). The global securities will be deposited with, or on behalf of, DTC and registered in the name of Cede & Co., the nominee of DTC. Unless and until it is exchanged for individual certificates evidencing securities under the limited circumstances described below, a global security may not be transferred except as a whole by the depositary to its nominee or by the nominee to the depositary, or by the depositary or its nominee to a successor depositary or to a nominee of the successor depositary.
DTC has advised us that it is:
| · | a limited-purpose trust company organized under the New York Banking Law; |
| · | a “banking organization” within the meaning of the New York Banking Law; |
| · | a member of the Federal Reserve System; |
| · | a “clearing corporation” within the meaning of the New York Uniform Commercial Code; and |
| · | a “clearing agency” registered pursuant to the provisions of Section 17A of the Exchange Act. |
| 24 |
DTC holds securities that its participants deposit with DTC. DTC also facilitates the settlement among its participants of securities transactions, such as transfers and pledges, in deposited securities through electronic computerized book-entry changes in participants’ accounts, thereby eliminating the need for physical movement of securities certificates. “Direct participants” in DTC include securities brokers and dealers, including underwriters, banks, trust companies, clearing corporations and other organizations. DTC is a wholly-owned subsidiary of The Depository Trust & Clearing Corporation (“DTCC”). DTCC is the holding company for DTC, National Securities Clearing Corporation and Fixed Income Clearing Corporation, all of which are registered clearing agencies. DTCC is owned by the users of its regulated subsidiaries. Access to the DTC system is also available to others, which we sometimes refer to as indirect participants, that clear through or maintain a custodial relationship with a direct participant, either directly or indirectly. The rules applicable to DTC and its participants are on file with the SEC.
Purchases of securities under the DTC system must be made by or through direct participants, which will receive a credit for the securities on DTC’s records. The ownership interest of the actual purchaser of a security, which we sometimes refer to as a beneficial owner, is in turn recorded on the direct and indirect participants’ records. Beneficial owners of securities will not receive written confirmation from DTC of their purchases. However, beneficial owners are expected to receive written confirmations providing details of their transactions, as well as periodic statements of their holdings, from the direct or indirect participants through which they purchased securities. Transfers of ownership interests in global securities are to be accomplished by entries made on the books of participants acting on behalf of beneficial owners. Beneficial owners will not receive certificates representing their ownership interests in the global securities, except under the limited circumstances described below.
To facilitate subsequent transfers, all global securities deposited by direct participants with DTC will be registered in the name of DTC’s partnership nominee, Cede & Co., or such other name as may be requested by an authorized representative of DTC. The deposit of securities with DTC and their registration in the name of Cede & Co. or such other nominee will not change the beneficial ownership of the securities. DTC has no knowledge of the actual beneficial owners of the securities. DTC’s records reflect only the identity of the direct participants to whose accounts the securities are credited, which may or may not be the beneficial owners. The participants are responsible for keeping account of their holdings on behalf of their customers.
So long as the securities are in book-entry form, you will receive payments and may transfer securities only through the facilities of the depositary and its direct and indirect participants. We will maintain an office or agency in the location specified in the prospectus supplement for the applicable securities, where notices and demands in respect of the securities and the indenture may be delivered to us and where certificated securities may be surrendered for payment, registration of transfer or exchange.
Conveyance of notices and other communications by DTC to direct participants, by direct participants to indirect participants and by direct participants and indirect participants to beneficial owners will be governed by arrangements among them, subject to any legal requirements in effect from time to time.
Redemption notices will be sent to DTC. If less than all of the securities of a particular series are being redeemed, DTC’s practice is to determine by lot the amount of the interest of each direct participant in the securities of such series to be redeemed.
Neither DTC nor Cede & Co. (or such other DTC nominee) will consent or vote with respect to the securities. Under its usual procedures, DTC will mail an omnibus proxy to us as soon as possible after the record date. The omnibus proxy assigns the consenting or voting rights of Cede & Co. to those direct participants to whose accounts the securities of such series are credited on the record date, identified in a listing attached to the omnibus proxy.
| 25 |
So long as securities are in book-entry form, we will make payments on those securities to the depositary or its nominee, as the registered owner of such securities, by wire transfer of immediately available funds. If securities are issued in definitive certificated form under the limited circumstances described below and unless if otherwise provided in the description of the applicable securities herein or in the applicable prospectus supplement, we will have the option of making payments by check mailed to the addresses of the persons entitled to payment or by wire transfer to bank accounts in the United States designated in writing to the applicable trustee or other designated party at least 15 days before the applicable payment date by the persons entitled to payment, unless a shorter period is satisfactory to the applicable trustee or other designated party.
Redemption proceeds, distributions and dividend payments on the securities will be made to Cede & Co., or such other nominee as may be requested by an authorized representative of DTC. DTC’s practice is to credit direct participants’ accounts upon DTC’s receipt of funds and corresponding detail information from us on the payment date in accordance with their respective holdings shown on DTC records. Payments by participants to beneficial owners will be governed by standing instructions and customary practices, as is the case with securities held for the account of customers in bearer form or registered in “street name.” Those payments will be the responsibility of participants and not of DTC or us, subject to any statutory or regulatory requirements in effect from time to time. Payment of redemption proceeds, distributions and dividend payments to Cede & Co., or such other nominee as may be requested by an authorized representative of DTC, is our responsibility; disbursement of payments to direct participants is the responsibility of DTC; and disbursement of payments to the beneficial owners is the responsibility of direct and indirect participants.
Except under the limited circumstances described below, purchasers of securities will not be entitled to have securities registered in their names and will not receive physical delivery of securities. Accordingly, each beneficial owner must rely on the procedures of DTC and its participants to exercise any rights under the securities and the indenture.
The laws of some jurisdictions may require that some purchasers of securities take physical delivery of securities in definitive form. Those laws may impair the ability to transfer or pledge beneficial interests in securities.
DTC may discontinue providing its services as securities depositary with respect to the securities at any time by giving reasonable notice to us. Under such circumstances, in the event that a successor depositary is not obtained, securities certificates are required to be printed and delivered.
As noted above, beneficial owners of a particular series of securities generally will not receive certificates representing their ownership interests in those securities. However, if:
| · | DTC notifies us that it is unwilling or unable to continue as a depositary for the global security or securities representing such series of securities or if DTC ceases to be a clearing agency registered under the Exchange Act at a time when it is required to be registered and a successor depositary is not appointed within 90 days of the notification to us or of our becoming aware of DTC’s ceasing to be so registered, as the case may be; |
| · | we determine, in our sole discretion, not to have such securities represented by one or more global securities; or |
| · | an Event of Default has occurred and is continuing with respect to such series of securities, |
we will prepare and deliver certificates for such securities in exchange for beneficial interests in the global securities. Any beneficial interest in a global security that is exchangeable under the circumstances described in the preceding sentence will be exchangeable for securities in definitive certificated form registered in the names that the depositary directs. It is expected that these directions will be based upon directions received by the depositary from its participants with respect to ownership of beneficial interests in the global securities.
| 26 |
We may sell the securities offered pursuant to this prospectus from time to time in one or more transactions, including, without limitation:
| · | to or through underwriters; |
| · | through broker-dealers (acting as agent or principal); |
| · | through agents; |
| · | directly by us to one or more purchasers (including our affiliates and stockholders), through a specific bidding or auction process, a rights offering or otherwise; |
| · | through a combination of any such methods of sale; or |
| · | through any other methods described in a prospectus supplement. |
The distribution of securities may be effected, from time to time, in one or more transactions, including:
| · | block transactions (which may involve crosses) and transactions on any national exchange or any other organized market where the securities may be traded; |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its own account pursuant to a prospectus supplement; |
| · | ordinary brokerage transactions and transactions in which a broker-dealer solicits purchasers; |
| · | sales “at the market” to or through a market maker or into an existing trading market, on an exchange or otherwise; and |
| · | sales in other ways not involving market makers or established trading markets, including direct sales to purchasers. |
The applicable prospectus supplement will describe the terms of the offering of the securities, including:
| · | the name or names of any underwriters, if, and if required, any dealers or agents; |
| · | the purchase price of the securities and the proceeds we will receive from the sale; |
| · | any underwriting discounts and other items constituting underwriters’ compensation; |
| · | any discounts or concessions allowed or re-allowed or paid to dealers; and |
| · | any securities exchange or market on which the securities may be listed or traded. |
We may distribute the securities from time to time in one or more transactions at:
| · | a fixed price or prices, which may be changed; |
| · | market prices prevailing at the time of sale; |
| · | prices related to such prevailing market prices; or |
| · | negotiated prices. |
Only underwriters named in the prospectus supplement are underwriters of the securities offered by the prospectus supplement.
| 27 |
If underwriters are used in an offering, we will execute an underwriting agreement with such underwriters and will specify the name of each underwriter and the terms of the transaction (including any underwriting discounts and other terms constituting compensation of the underwriters and any dealers) in a prospectus supplement. The securities may be offered to the public either through underwriting syndicates represented by managing underwriters or directly by one or more investment banking firms or others, as designated. If an underwriting syndicate is used, the managing underwriter(s) will be specified on the cover of the prospectus supplement. If underwriters are used in the sale, the offered securities will be acquired by the underwriters for their own accounts and may be resold from time to time in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. Any public offering price and any discounts or concessions allowed or re-allowed or paid to dealers may be changed from time to time. Unless otherwise set forth in the prospectus supplement, the obligations of the underwriters to purchase the offered securities will be subject to conditions precedent, and the underwriters will be obligated to purchase all of the offered securities, if any are purchased.
We may grant to the underwriters options to purchase additional securities to cover over-allotments, if any, at the public offering price, with additional underwriting commissions or discounts, as may be set forth in a related prospectus supplement. The terms of any over-allotment option will be set forth in the prospectus supplement for those securities.
If a dealer is used in the sale of the securities, we, or an underwriter, will sell the securities to the dealer, as principal. The dealer may then resell the securities to the public at varying prices to be determined by the dealer at the time of resale. To the extent required, we will set forth in the prospectus supplement, document incorporated by reference or free writing prospectus, as applicable, the name of the dealer and the terms of the transactions.
We may sell the securities directly or through agents we designate from time to time. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the prospectus supplement.
We may authorize agents or underwriters to solicit offers by institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
In connection with the sale of the securities, underwriters, dealers or agents may receive compensation from us or from purchasers of the securities for whom they act as agents, in the form of discounts, concessions or commissions. Underwriters may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers for whom they may act as agents. Underwriters, dealers and agents that participate in the distribution of the securities, and any institutional investors or others that purchase securities directly for the purpose of resale or distribution, may be deemed to be underwriters, and any discounts or commissions received by them from us and any profit on the resale of the common stock by them may be deemed to be underwriting discounts and commissions under the Securities Act. No FINRA member firm may receive compensation in excess of that allowable under FINRA rules, including Rule 5110, in connection with the offering of the securities.
| 28 |
We may provide agents, underwriters and other purchasers with indemnification against particular civil liabilities, including liabilities under the Securities Act, or contribution with respect to payments that the agents, underwriters or other purchasers may make with respect to such liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business.
To facilitate the public offering of a series of securities, persons participating in the offering may engage in transactions that stabilize, maintain, or otherwise affect the market price of the securities. This may include over-allotments or short sales of the securities, which involves the sale by persons participating in the offering of more securities than have been sold to them by us. In addition, those persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to underwriters or dealers participating in any such offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. Such transactions, if commenced, may be discontinued at any time. We make no representation or prediction as to the direction or magnitude of any effect that the transactions described above, if implemented, may have on the price of our securities.
Unless otherwise specified in the applicable prospectus supplement, any common stock sold pursuant to a prospectus supplement will be eligible for trading as quoted on the OTCQB. Any underwriters to whom securities are sold by us for public offering and sale may make a market in the securities, but such underwriters will not be obligated to do so and may discontinue any market making at any time without notice.
In order to comply with the securities laws of some states, if applicable, the securities offered pursuant to this prospectus will be sold in those states only through registered or licensed brokers or dealers. In addition, in some states securities may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and complied with.
To the extent required, this prospectus may be amended or supplemented from time to time to describe a specific plan of distribution.
| 29 |
Certain legal matters governed by New York law with respect to the validity of certain of the offered securities will be passed upon for us by Troutman Pepper Hamilton Sanders LLP, New York, New York. Certain legal matters governed by Nevada law with respect to the validity of certain of the offered securities will be passed upon for us by Ballard Spahr LLP, Las Vegas, Nevada.
Armanino LLP, independent registered public accounting firm, has audited our consolidated financial statements included in our Annual Report on Form 10-K for the year ended April 30, 2020, as set forth in their report, which is incorporated by reference in the prospectus and elsewhere in this registration statement. Our consolidated financial statements are incorporated by reference in reliance on Armanino LLP’s report, given on their authority as experts in accounting and auditing.
| 30 |
WHERE YOU CAN FIND MORE INFORMATION
This prospectus and any subsequent prospectus supplements do not contain all of the information in the Registration Statement. We have omitted from this prospectus some parts of the Registration Statement as permitted by the rules and regulations of the SEC. Statements in this prospectus concerning any document we have filed as an exhibit to the Registration Statement or that we otherwise filed with the SEC are not intended to be comprehensive and are qualified in their entirety by reference to these filings. In addition, we file annual, quarterly and current reports and other information with the SEC. The SEC maintains a website that contains reports and information statements and other information that registrants file electronically with the SEC, including us. The SEC’s website can be found at http://www.sec.gov. In addition, we make available on or through our website copies of these reports as soon as reasonably practicable after we electronically file or furnished them to the SEC. Our website can be found at http:www.pharmacyte.com. Our website is not a part of this prospectus.
INFORMATION INCORPORATED BY REFERENCE
We have elected to incorporate certain information by reference into this prospectus. By incorporating by reference, we can disclose important information to you by referring you to other documents we have filed or will file with the SEC. The information incorporated by reference is deemed to be part of this prospectus, except for information incorporated by reference that is superseded by information contained in this prospectus. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any statements in the prospectus or any document previously incorporated by reference have been modified or superseded. This prospectus incorporates by reference the documents set forth below that we have previously filed with the SEC, except in each case the information contained in such document to the extent “furnished” and not “filed”:
| · | Our Annual Report on Form 10-K for the fiscal year ended April 30, 2020, filed with the SEC on August 13, 2020. |
| · | Our Quarterly Report on Form 10-Q for the quarterly period ended July 31, 2020, filed with the SEC on September 11, 2020. |
| · | Our Quarterly Report on Form 10-Q for the quarterly period ended October 31, 2020, filed with the SEC on December 11, 2020. |
| · | Our Quarterly Report on Form 10-Q for the quarterly period ended January 31, 2021, filed with the SEC on March 12, 2021. |
| · | Our Current Report on Form 8-K filed with the SEC on October 16, 2020. |
We also incorporate by reference all documents we file in the future pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act after the date of this prospectus and prior to the sale of all the securities covered by this prospectus (including all such documents filed with the SEC after the date of the initial filing of the Registration Statement that contains this prospectus and prior to effectiveness of the Registration Statement or after such effectiveness), except in each case the information contained in such document to the extent “furnished” and not “filed.”
You may obtain copies of these documents on the website maintained by the SEC at http://www.sec.gov, or from us without charge (other than exhibits to such documents, unless such exhibits are specifically incorporated by reference into such documents) by writing us at Corporate Secretary, PharmaCyte Biotech, Inc., 23046 Avenida de la Carlota, Suite 600, Laguna Hills, California 92653 or visiting our website at http://www.pharmacyte.com.
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus shall be deemed to be modified or superseded for the purposes of this prospectus to the extent that a statement contained herein, any prospectus supplement or in any other subsequently filed document which also is or deemed to be incorporated by reference herein modifies or supersedes that statement. Any statement so modified or superseded shall not be deemed, except as so modified or superseded, to constitute a part of this prospectus.
| 31 |

Shares of Common Stock
Pre-funded Warrants to Purchase up to Shares of Common Stock
Common Warrants to Purchase up to Shares of Common Stock
Underwriter Warrants to Purchase Shares of Common Stock
PROSPECTUS SUPPLEMENT
Sole Book-Running Manager
H.C. WAINWRIGHT & CO.
, 2021